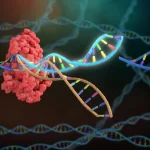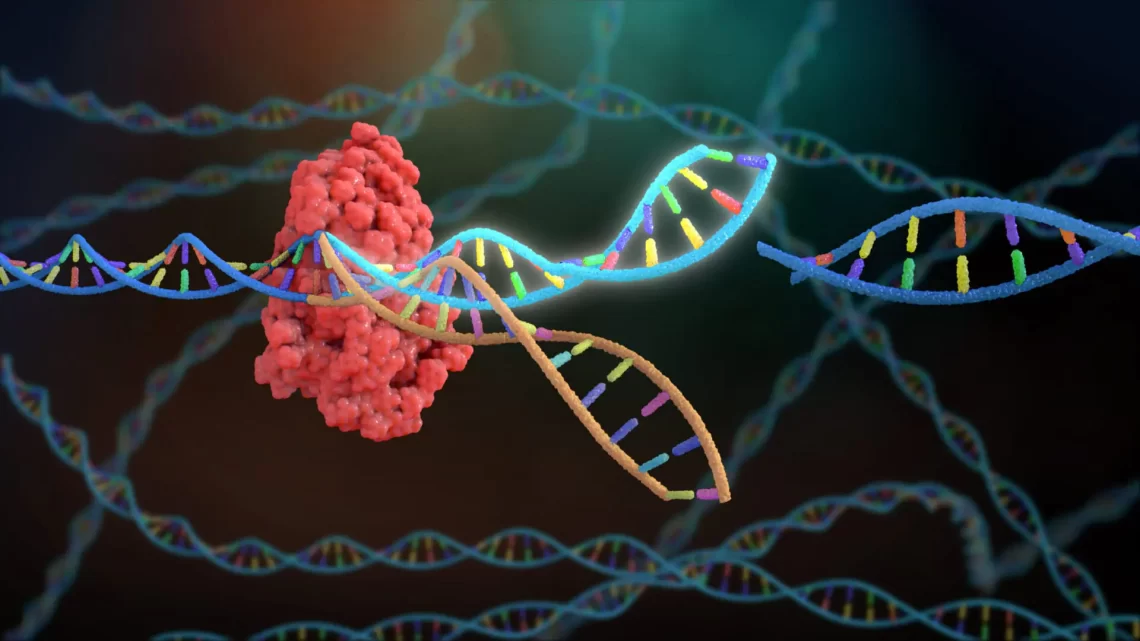
CRISPR Beyond DNA: Expanding Applications in RNA Editing, Epigenetics, and Non-Coding RNA Targeting
February 21, 2025The revolutionary CRISPR-Cas system, initially recognized for its ability to precisely edit DNA, has now evolved far beyond simple genome modification. Researchers have adapted CRISPR technology to target RNA, modify epigenetic marks, and regulate non-coding RNAs, significantly broadening its potential applications in both research and therapeutic contexts. This expansion of CRISPR technology has profound implications for understanding gene expression, treating diseases, and developing novel biotechnological tools.
1. RNA Editing with CRISPR-Cas Systems
While traditional CRISPR-Cas9 primarily focuses on DNA editing, alternative CRISPR-associated proteins such as Cas13 have been developed to specifically target RNA. Unlike Cas9, which creates double-strand breaks in DNA, Cas13 is an RNA-targeting nuclease that can be programmed to recognize and cleave specific RNA sequences without affecting the genome.
Key Applications of CRISPR-Based RNA Editing:
- Correction of RNA Mutations: CRISPR-Cas13 systems enable precise modifications at the RNA level, offering a transient and reversible alternative to DNA editing. This is particularly useful for treating genetic disorders where permanent genomic changes might be risky.
- Viral RNA Targeting: CRISPR-Cas13 has been explored for antiviral strategies, particularly against RNA viruses such as SARS-CoV-2. By targeting and degrading viral RNA, CRISPR-based therapies could suppress infection and replication.
- Regulating Gene Expression: By degrading or modifying messenger RNA (mRNA), CRISPR-Cas13 can control protein synthesis, making it a powerful tool for studying gene function and developing gene therapy approaches.
2. CRISPR for Epigenetic Modifications
CRISPR-Cas9 has also been repurposed for epigenetic editing, enabling researchers to modify DNA methylation and histone modifications without altering the genetic sequence. This is achieved using catalytically dead Cas9 (dCas9), which binds to DNA but does not induce cuts, allowing it to act as a guide for epigenetic enzymes.
Key Approaches to CRISPR-Based Epigenetic Editing:
- DNA Methylation/Demethylation: Fusion of dCas9 with DNA methyltransferases or demethylases allows researchers to control gene silencing or activation by modifying cytosine methylation.
- Histone Modification: dCas9 can be linked to histone-modifying enzymes, such as histone acetyltransferases or deacetylases, to regulate chromatin accessibility and gene expression.
- Reversible Gene Regulation: Unlike traditional gene editing, epigenetic modifications do not permanently alter the genome, making them ideal for reversible therapies in diseases such as cancer, neurological disorders, and metabolic conditions.
Therapeutic Implications:
- Neurological Disorders: Epigenetic modifications via CRISPR have been proposed for treating diseases like Alzheimer’s and Huntington’s by reactivating silenced genes or suppressing toxic gene expression.
- Cancer Therapy: CRISPR-based epigenetic regulation can switch off oncogenes or reactivate tumor suppressor genes without introducing permanent genetic changes.
- Metabolic Diseases: Epigenetic CRISPR tools could be used to modify gene expression patterns linked to obesity, diabetes, and other metabolic disorders.
3. Targeting Non-Coding RNAs (ncRNAs) with CRISPR
Non-coding RNAs (ncRNAs) such as long non-coding RNAs (lncRNAs) and microRNAs (miRNAs) play critical roles in gene regulation, but they have been challenging to study and manipulate due to their lack of direct protein-coding function. CRISPR-based tools now allow precise modulation of these RNA molecules.
Applications of CRISPR in ncRNA Research and Therapy:
- CRISPR-Cas13 for lncRNA Knockdown: By cleaving lncRNAs, CRISPR-Cas13 enables researchers to study their function in various biological processes, including cancer, neurodevelopment, and immune response.
- miRNA Editing and Regulation: CRISPR can be used to either block or enhance miRNA activity, influencing gene expression networks involved in diseases such as cardiovascular disorders and neurodegeneration.
- Therapeutic Targeting of Disease-Associated ncRNAs: Many ncRNAs are implicated in diseases, making them attractive therapeutic targets. CRISPR-based RNA editing could silence pathogenic lncRNAs or restore normal miRNA functions.
Future Perspectives and Challenges
While CRISPR applications beyond DNA editing hold great promise, several challenges remain:
- Delivery Mechanisms: Efficiently delivering CRISPR-based RNA or epigenetic editors into cells and tissues remains a significant hurdle for clinical applications. Viral vectors, nanoparticles, and direct RNA delivery methods are being explored.
- Off-Target Effects: Ensuring precise targeting with minimal unintended modifications is crucial for safe therapeutic applications.
- Ethical and Regulatory Considerations: As CRISPR moves beyond the laboratory into clinical settings, ethical concerns regarding gene regulation, reversibility, and unintended consequences must be carefully addressed.
Conclusion
The adaptation of CRISPR technology beyond DNA editing is transforming biomedical research and therapy. RNA editing with Cas13 offers dynamic gene regulation without permanent DNA alterations, epigenetic modifications allow for reversible control of gene expression, and targeting ncRNAs opens new avenues for understanding and treating diseases. As CRISPR tools continue to evolve, their applications in medicine, biotechnology, and synthetic biology will expand, paving the way for innovative treatments and a deeper understanding of cellular processes.