Exome: The Promises and Pitfalls of Whole Exome Sequencing
October 24, 2023Table of Contents
1. Introduction: The Evolution of Genetic Sequencing
Brief Overview of Genomics and its Significance
Genomics, a discipline in genetics, concerns itself with the study of the genomes of organisms. The genome is the entire set of DNA in an organism, including all of its genes. Each piece of DNA contains information, much like a set of instructions, that determines everything from the color of our eyes to our susceptibility to certain diseases.
The significance of genomics is vast:
- Disease Understanding and Treatment: By studying the human genome, scientists have been able to identify genes associated with certain diseases, leading to better diagnostics, treatments, and even cures.
- Personalized Medicine: Knowledge of an individual’s genomic sequence can allow for tailored medical treatments, ensuring that the prescribed medicine is the most effective and safest for each unique genetic makeup.
- Evolutionary Insights: By comparing genomes across various species, we gain insight into evolutionary processes and the relatedness of different organisms.
- Agriculture and Biodiversity: In non-human genomics, understanding the genetic makeup of plants and animals can lead to improved crop varieties and protection of endangered species.
Introduction to the Concept of the Exome
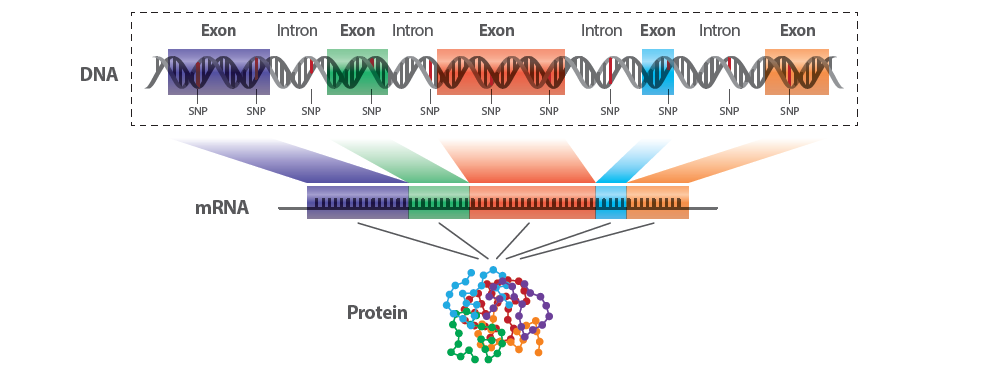
The genome is vast, but not all of it is equally understood or equally active in producing proteins, the workhorses of the cell. Roughly 1-2% of the human genome is made up of regions known as exons which code for proteins. The complete set of these protein-coding regions is called the exome.
Studying the exome, often through a process known as whole exome sequencing, is of particular interest for several reasons:
- Disease Association: Many known genetic mutations that lead to diseases are located in the exons. By focusing on the exome, researchers can often find these mutations more quickly and at a lower cost than sequencing the entire genome.
- Efficiency: Instead of looking at all 3 billion base pairs in the human genome, scientists can focus on the 30 million base pairs in the exome, making the process quicker and more focused.
- Protein Alterations: Since the exome contains the information needed to make proteins, changes in the exome can lead to changes in proteins, which can subsequently affect health and disease states.
In conclusion, as genetic sequencing technologies have evolved, they’ve provided unprecedented insights into the human genome and its implications for health, disease, and our broader understanding of life. The focus on the exome, in particular, has become a powerful tool in this exploration, offering a targeted approach to uncovering the genetic basis of many conditions.
2. What is Whole Exome Sequencing (WES)?
Definition and Process of WES:
Whole Exome Sequencing (WES) is a genomic technique that specifically sequences the protein-coding regions of genes, known as exons. As mentioned earlier, the exome represents approximately 1-2% of the entire genome but contains about 85% of known disease-related mutations, making it a prime target for research.
The basic process of WES includes:
- DNA Extraction: DNA is first extracted from a sample, which can be sourced from blood, saliva, or tissue.
- Library Preparation: The extracted DNA is then fragmented and special adapters are attached to the ends of these fragments.
- Exome Capture: Using probes or “baits” that match known exon sequences, the exonic regions are selectively pulled out or “captured” from the fragmented DNA. This is a crucial step that differentiates WES from other sequencing methods.
- Sequencing: The captured exonic DNA fragments are then sequenced using high-throughput sequencing platforms.
- Data Analysis: The sequence data generated is aligned to a reference genome and analyzed for variants. Variants, especially in the coding region, are then studied further for their potential association with diseases or traits.
Comparison to Whole Genome Sequencing (WGS):
- Coverage: While WES targets only the exonic regions (1-2% of the genome), WGS sequences nearly the entire genome, including both coding and non-coding regions.
- Cost and Speed: WES is typically cheaper and faster than WGS due to its targeted approach. WGS, on the other hand, requires sequencing and analyzing a much larger amount of DNA.
- Resolution: WGS offers a more comprehensive view of the genome and can detect a wider range of genetic variations, including those in non-coding regions, which might have regulatory, structural, or other functions.
- Clinical Relevance: While WES captures a majority of known disease-associated mutations found in the coding regions, WGS can identify mutations in regulatory regions, introns, and other non-coding regions that might also have clinical relevance.
- Application: WES is often preferred when the focus is on identifying mutations in the coding regions, especially for rare genetic disorders. WGS is chosen when a comprehensive view of the genome is required, especially for complex diseases where non-coding regions might play a crucial role or for research purposes.
In summary, both WES and WGS have their own strengths and are chosen based on the specific needs of the study. While WES offers a targeted and cost-effective approach to identify variants in protein-coding regions, WGS provides a broader view of the entire genome, capturing all types of genetic variations.
3. Promises of Whole Exome Sequencing
a. Cost-Effective Analysis
- Price Comparison with Other Sequencing Methods: Whole Exome Sequencing (WES) is considerably more cost-effective than Whole Genome Sequencing (WGS) because it targets only about 1-2% of the genome. By focusing on the exome, researchers can significantly reduce the costs associated with sequencing, data storage, and analysis.
- Potential for Making Sequencing More Accessible: Because of its cost-effectiveness, WES has the potential to democratize genetic testing, making it available to a wider audience. This can lead to more people having access to genetic information, paving the way for broader public health initiatives and more inclusive research.
b. Rapid Identification of Disease-Causing Variants
- Case Studies Showcasing Success: There have been numerous instances where WES has identified causative mutations for rare genetic disorders. For example, families with unidentified genetic conditions have found answers through WES after years of inconclusive tests, a journey often referred to as the “diagnostic odyssey.”
- Importance in Rare Disease Diagnosis: Many rare diseases are caused by mutations in protein-coding regions. WES, therefore, plays a crucial role in diagnosing these conditions, providing answers to patients and potentially guiding treatment options.
c. Personalized Medicine
- Pharmacogenomics: Tailoring Treatments Based on Genetic Makeup: Variants in the exome can affect how individuals respond to drugs. By analyzing these variants, doctors can prescribe medications that are more effective and carry fewer side effects for a particular individual. This is the essence of pharmacogenomics.
- Predictive Medicine: Identifying Risks and Prevention Strategies: WES can identify variants associated with an increased risk of certain diseases. By knowing these risks, individuals can take preventive measures, and healthcare providers can offer more targeted screenings.
d. Advances in Research
- Boosting Research on Protein-Coding Regions of the Genome: By focusing on the exome, researchers can delve deeper into the function of proteins, how they interact, and how changes in their coding can lead to diseases. This can lead to a richer understanding of biology and disease mechanisms.
- Contribution to the Discovery of New Genes and Associated Functions: WES has been instrumental in the discovery of previously unknown genes and their functions. By analyzing the exome data of individuals from diverse populations, researchers can identify new genes, discern their functions, and understand their role in health and disease.
In essence, the promises of Whole Exome Sequencing are manifold. From cost-effective analyses to advances in research, WES is ushering in a new era in genomics, heralding significant benefits for patients, researchers, and the broader medical community.
4. Pitfalls and Challenges of WES
a. Incomplete Coverage
- Regions of the Exome That Might Not Be Captured: Even though WES is designed to capture the entirety of the exome, there are regions that might not be efficiently captured due to their repetitive nature, high GC content, or other genomic complexities. As a result, some mutations in these areas might be missed.
- Importance of Considering Sequencing Depth: Sequencing depth refers to the number of times a particular base is read during the sequencing process. A higher depth increases the confidence in the detected variants. However, uneven sequencing depth across the exome can lead to regions being under-sequenced, possibly missing out on significant variants.
b. Interpretation Difficulties
- Challenges in Differentiating Between Benign and Harmful Variants: Not all mutations result in disease. Distinguishing benign variations from those that are harmful or disease-causing can be challenging, especially with rare or previously unidentified variants.
- Current State of Databases and Annotation Tools: While databases and annotation tools have significantly improved over the years, they are not infallible. They might lack information on certain rare variants, leading to challenges in accurate interpretation.
- Unintended Discoveries and Incidental Findings: WES can reveal mutations that were not the primary target of the test, such as predispositions to late-onset diseases. Handling and communicating these incidental findings can be ethically challenging.
- Patient Consent and Genetic Counseling Considerations: It’s essential that patients understand what WES can and cannot offer, as well as the potential implications of the results. Informed consent and post-test genetic counseling become paramount in ensuring that patients are adequately prepared and supported.
d. Technical Limitations
- Possible Errors and Artifacts in the Sequencing Process: Like any technology, WES is prone to errors. These can arise from sample preparation, sequencing, or data processing stages. Artifacts or false positives/negatives can sometimes be introduced.
- Limitation in Detecting Structural Variants or Epigenetic Changes: WES is optimized for detecting single nucleotide variants and small insertions or deletions. It’s less effective in detecting larger structural variants, like large deletions, duplications, or chromosomal rearrangements. Additionally, WES doesn’t provide information on epigenetic changes, which can also play a role in disease.
In conclusion, while Whole Exome Sequencing offers numerous promises and has revolutionized genetic research and clinical diagnostics, it’s essential to be aware of its limitations and challenges. This awareness ensures that results are interpreted accurately, ethically, and in the best interest of the patient or research objective.
5. Future Directions and Innovations
Whole Exome Sequencing (WES), like many other genomic technologies, is not static. It’s rapidly evolving, with future directions influenced by technological advancements, integration possibilities, and computational innovations.
Technological Improvements and Their Implications:
- Higher Resolution and Accuracy: As sequencing technologies advance, we can expect even more accurate reads with fewer errors. This would allow for more confident detection of mutations and a reduction in false positives/negatives.
- Enhanced Exome Capture: Improvements in the exome capture techniques might lead to better coverage of traditionally hard-to-sequence regions, ensuring that fewer mutations are missed.
- Lower Costs: The trend in genomic sequencing has consistently been a decrease in costs. As technologies become more efficient and scalable, WES might become even more accessible to the broader population.
Integration with Other Technologies and Data Types (like Transcriptomics):
- Multi-Omics Approach: There’s a growing interest in integrating genomic data (like WES) with other “omics” data, such as transcriptomics (RNA sequencing), proteomics, and metabolomics. Such an integrated approach can offer a more comprehensive view of biological processes and disease mechanisms.
- Functional Validation: Future research might see a closer integration of WES with functional assays, where identified mutations are experimentally tested for their effects on cellular processes, enhancing our understanding of their roles in diseases.
The Potential Role of AI and Machine Learning in Data Interpretation:
- Variant Interpretation: AI models can be trained to predict the functional impact of genetic variants, differentiating between benign and potentially harmful mutations. This is especially valuable for variants that are rare or previously uncharacterized.
- Pattern Recognition: Machine learning algorithms excel at recognizing patterns within vast datasets. In the context of WES, this could mean identifying combinations of mutations that collectively contribute to a disease, especially for complex conditions where multiple genes might be involved.
- Integration of Multiple Data Types: AI can help in integrating WES data with other data types, like clinical data, imaging, or other omics data, providing a holistic view of a patient’s health and potential disease risks.
- Continuous Learning and Database Improvement: As more WES data becomes available, AI can assist in continuously updating and refining databases and annotation tools, ensuring that they are always up-to-date with the latest discoveries.
In summary, the future of Whole Exome Sequencing is bright, with numerous innovations on the horizon. These advancements, especially when combined with computational power and AI, have the potential to revolutionize how we understand, diagnose, and treat diseases.
6. Conclusion: The Balance of Potential and Prudence
Whole Exome Sequencing (WES) stands at the forefront of genomic medicine, holding vast potential to transform our understanding of the genetic underpinnings of disease. Its power to pinpoint disease-causing mutations, especially in the realm of rare disorders, heralds a new era of diagnosis, treatment, and prevention. With its cost-effectiveness and specificity, WES democratizes access to genetic information, allowing more individuals to benefit from the insights of genomic medicine.
However, with great potential comes great responsibility. The very capabilities that make WES a promising tool also bring forth challenges. The intricacies of the human genome mean that even as we sequence the exome, there are regions we might miss or misinterpret. Furthermore, the discovery of unintended genetic findings can pose ethical dilemmas, especially when the implications of these findings are uncertain.
Therefore, it’s crucial to approach the promises of WES with prudence. Recognizing its limits is just as important as heralding its capabilities. In the quest to harness the full potential of WES, there must be a continued emphasis on refining technologies, enhancing databases, and ensuring accurate interpretation of results.
A balanced approach to WES also requires an ongoing commitment to ethical considerations. As researchers and clinicians, there’s a duty to ensure that genetic information is handled with the utmost respect and sensitivity. This means providing comprehensive genetic counseling, ensuring informed consent, and navigating the challenges of incidental findings with care.
Lastly, patient education remains paramount. As the beneficiaries of WES, patients have the right to fully understand the process, its potential outcomes, and its limitations. Empowered with knowledge, they can make informed decisions about their health, advocate for their needs, and contribute to a future where genomic medicine is practiced with both potential and prudence in mind.
In conclusion, Whole Exome Sequencing, with its myriad of promises, stands as a beacon of what’s possible in modern medicine. Yet, it’s only by recognizing its challenges, and by approaching it with care, consideration, and continuous research, that we can truly harness its transformative power for the betterment of all.


