The Largest Whole-genome Sequencing Study in Cancer Unveils Potential Treatment and Prognostic Insights
April 18, 2024A groundbreaking study published in Nature Medicine conducted comprehensive whole-genome sequencing (WGS) of 13,880 tumors, shedding light on somatic and germline mutations that could significantly impact patient treatment strategies and outcomes. This study underscores the importance of comprehensive cancer genomics in shaping personalized medicine approaches.
Key Findings and Implications:
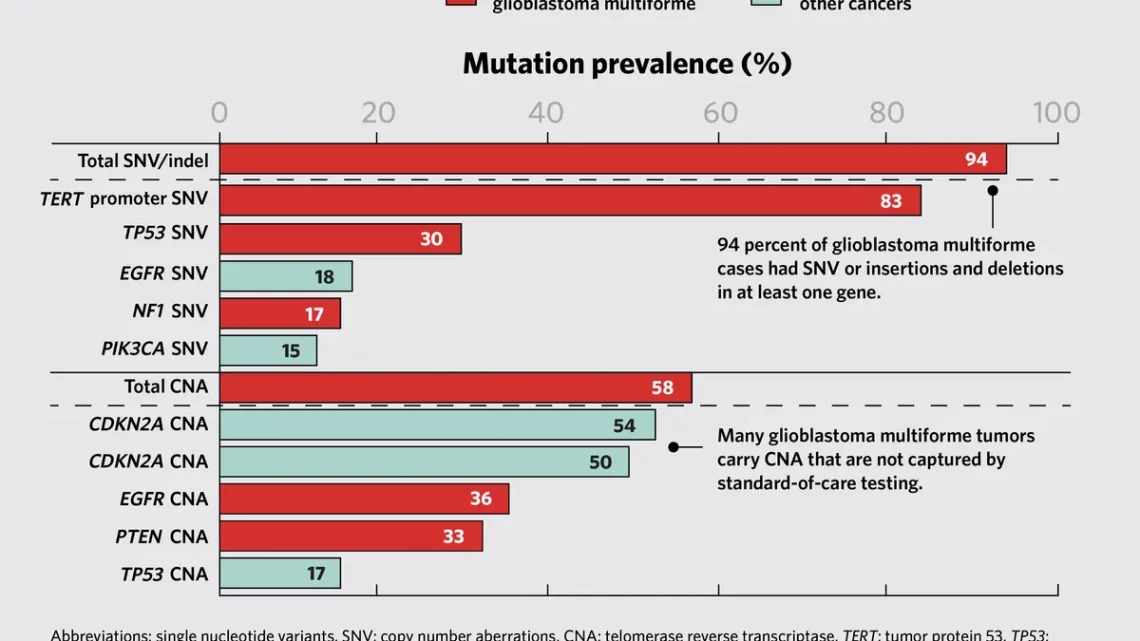
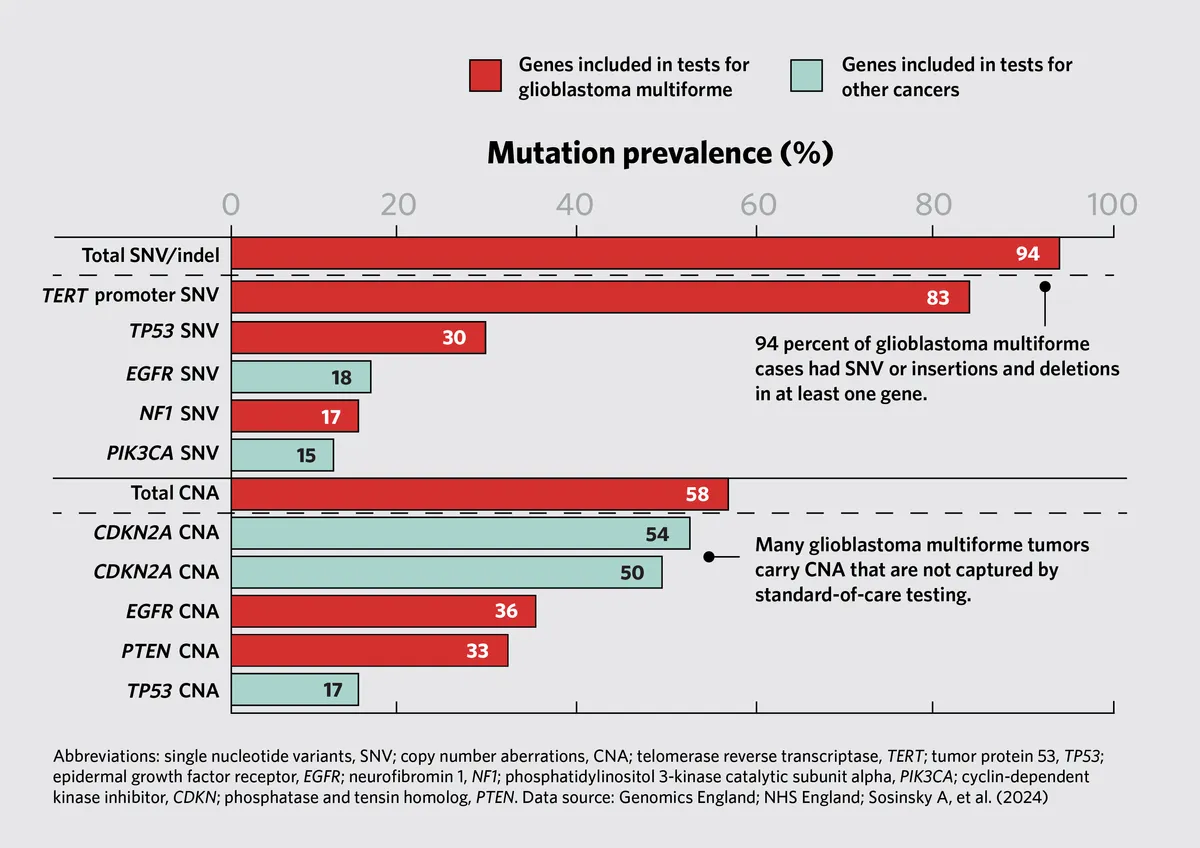
- Complex Genetic Landscape: Cancer is driven by a unique combination of genetic mutations, making each tumor genetically distinct. Standard targeted panels may not capture the full spectrum of genetic alterations, potentially limiting treatment options.
- Improved Patient Stratification: WGS revealed a wide range of somatic and germline mutations across different cancer types, highlighting the need for a more holistic approach to genetic testing. This could lead to better patient stratification and personalized treatment plans.
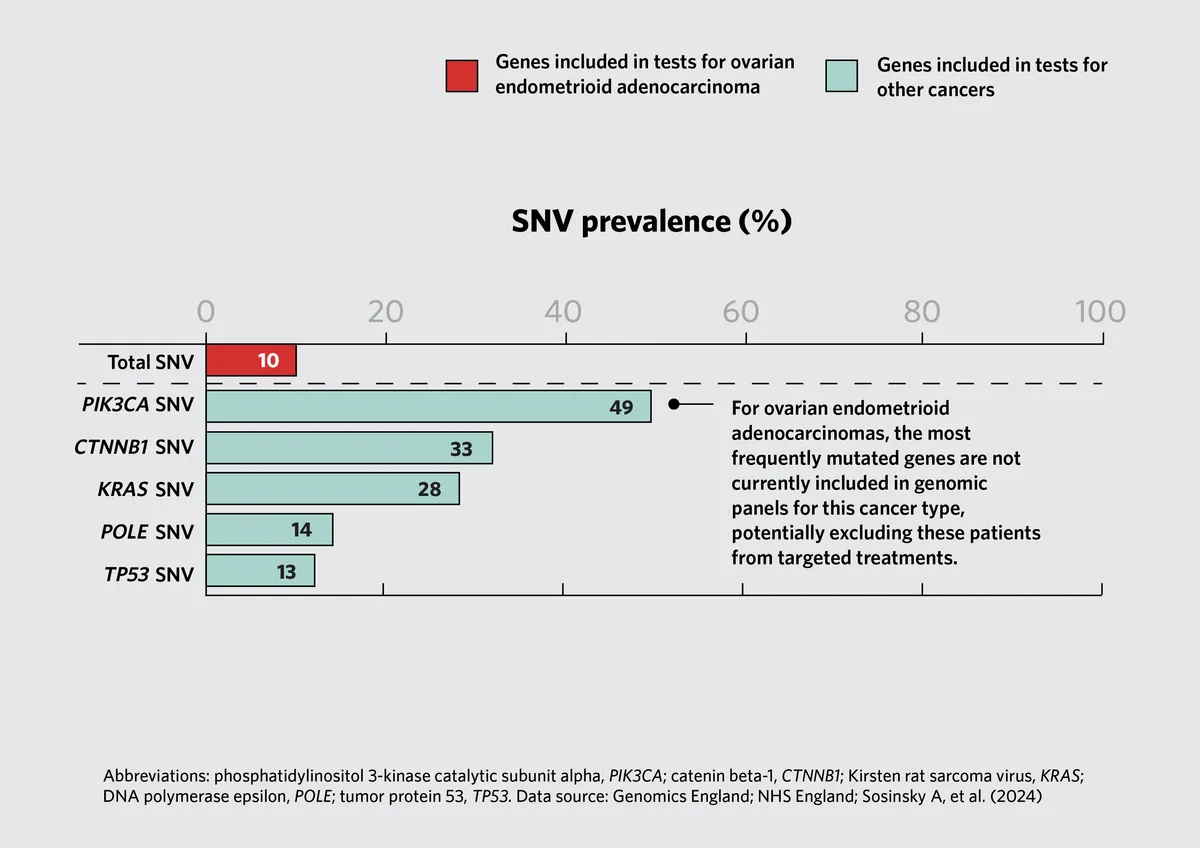
- Therapeutic Implications: The study identified mutations in genes not typically included in standard-of-care testing protocols. For example, mutations in the PIK3CA gene were found in uterine and ovarian cancers, suggesting potential therapeutic targets for clinical trials.
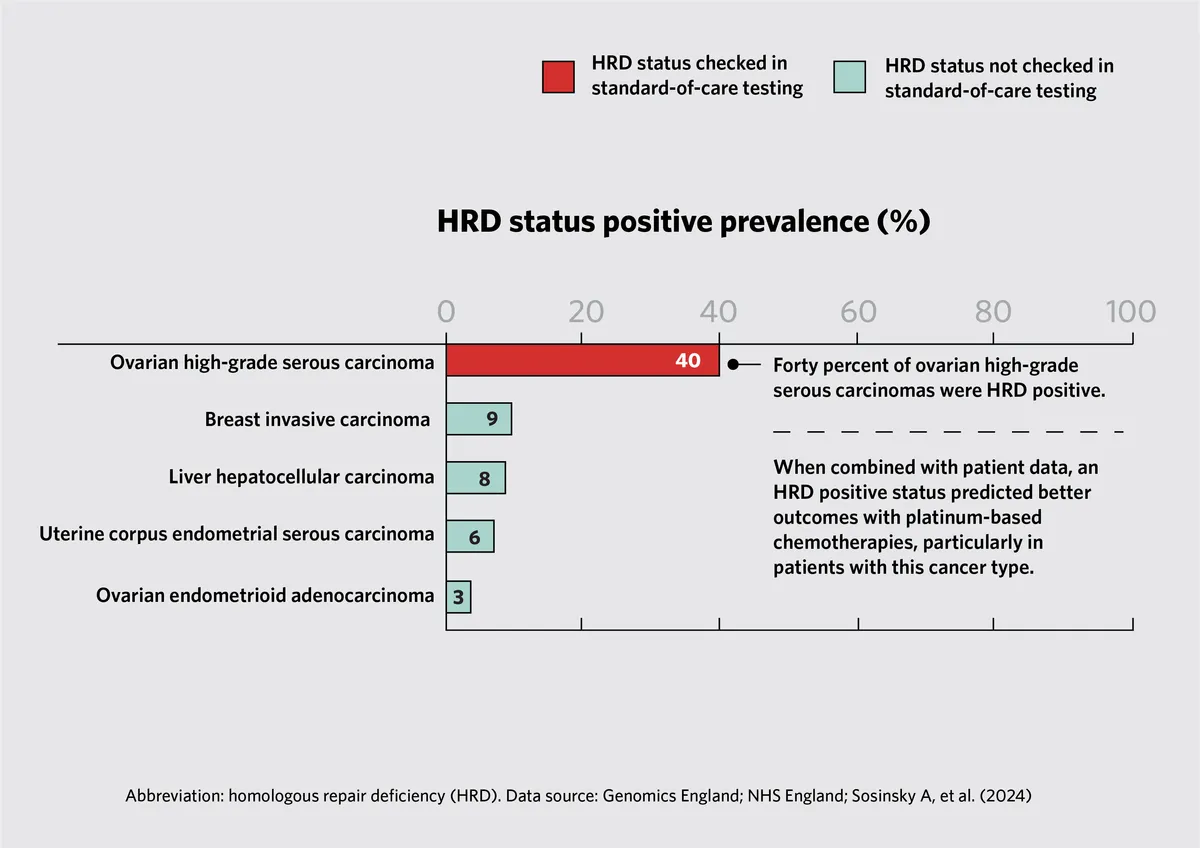
- Prognostic Insights: Analysis of genomic data alongside clinical outcomes revealed that patients with specific genetic alterations, such as homologous recombination deficiency (HRD), may benefit more from certain chemotherapies. This highlights the potential of genomic data in predicting treatment response and patient outcomes.
- Future Directions: While the study focused on WGS, future research could integrate other omics data, such as RNA sequencing, to gain a more comprehensive understanding of cancer biology. The ultimate goal is to leverage genomic data to guide precision oncology approaches and improve patient outcomes.
Impact of the Study:
- Advancing Precision Oncology: The study’s findings have significant implications for the future of cancer care, paving the way for more precise and effective treatment strategies based on individual genetic profiles.
- Cost Considerations: While the cost of whole-genome sequencing has decreased, it remains a limiting factor for widespread clinical adoption. However, researchers are hopeful that as costs continue to decline, WGS will become a more accessible and cost-effective tool for personalized cancer care.
- Clinical Trial Integration: The next phase of research involves integrating WGS data into clinical trials to identify biomarkers for treatment response. This could revolutionize the way clinical trials are conducted, providing valuable insights into treatment efficacy and patient outcomes.
Overall, the study represents a significant milestone in cancer genomics, highlighting the potential of comprehensive WGS in guiding personalized cancer treatment and improving patient outcomes.
REFERENCE
Sosinsky A, et al. Insights for precision oncology from the integration of genomic and clinical data of 13,880 tumors from the 100,000 Genomes Cancer Programme. Nat Med. 2024;30(1):279-289.


