Mesothelioma: A Bioinformatics Perspective on Diagnosis, Prognosis, and Treatment
November 28, 2023Table of Contents
I. Introduction
Mesothelioma research stands at the intersection of cutting-edge science and the urgent need for effective strategies in addressing this challenging cancer. Bioinformatics, a pivotal discipline, plays a crucial role in unraveling the complexities of mesothelioma. This introduction provides an overview of the indispensable role of bioinformatics in advancing our understanding of mesothelioma, from deciphering its genomic landscape to guiding precision medicine approaches.
1. Understanding Mesothelioma:
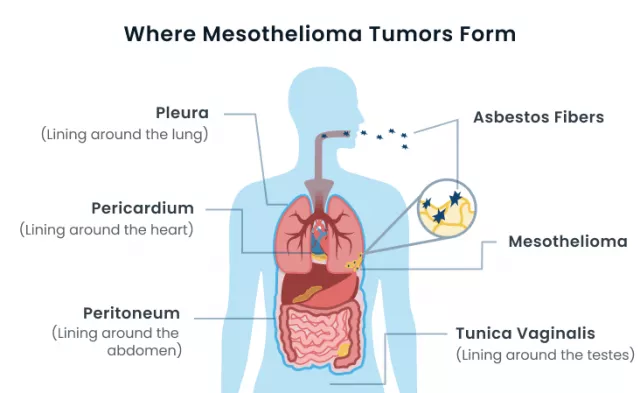
- Nature of the Disease: Mesothelioma, a deadly cancer primarily linked to asbestos exposure, poses unique challenges due to its aggressive nature and limited treatment options.
- Gaps in Knowledge: The mysteries surrounding why some individuals develop mesothelioma and the intricacies of its molecular underpinnings highlight the need for comprehensive research.
2. The Role of Bioinformatics:
- Unraveling Genomic Complexity: Bioinformatics serves as the cornerstone for deciphering the genomic complexity of mesothelioma, identifying key genetic mutations, and understanding chromosomal alterations.
- Integration of Omics Data: By integrating data from genomics, transcriptomics, proteomics, and other omics disciplines, bioinformatics provides a holistic view of the molecular landscape, revealing insights into disease progression.
3. Overcoming Challenges with Computational Tools:
- Handling Big Data: The massive volumes of data generated in mesothelioma research, especially with high-throughput technologies, necessitate advanced computational tools for efficient storage, analysis, and interpretation.
- Addressing Tumor Heterogeneity: Bioinformatics techniques play a pivotal role in addressing tumor heterogeneity, allowing researchers to capture the diverse molecular profiles within and across mesothelioma tumors.
4. Guiding Precision Medicine Approaches:
- Personalized Treatment Strategies: Bioinformatics facilitates the identification of genetic variations, biomarkers, and therapeutic targets, laying the groundwork for personalized and targeted treatment strategies.
- Pharmacogenomics and Drug Response: Understanding how individual genetic variations influence responses to chemotherapy, a realm explored through bioinformatics, informs decisions on drug selection and dosage.
5. Aims of Mesothelioma Research:
- Early Detection and Diagnosis: Bioinformatics contributes to the development of tools for early detection and accurate diagnosis, crucial for improving outcomes in mesothelioma.
- Innovative Therapies: By uncovering novel therapeutic targets and pathways, bioinformatics propels the exploration of innovative therapies for mesothelioma beyond conventional approaches.
Conclusion:
In the landscape of mesothelioma research, bioinformatics emerges as an indispensable ally, guiding researchers through the intricate molecular terrain of this challenging cancer. As advancements in genomic technologies and computational methodologies continue, the role of bioinformatics is poised to catalyze breakthroughs, offering hope for improved diagnostics, innovative treatments, and ultimately, better outcomes for individuals affected by mesothelioma. This overview sets the stage for a deeper exploration of specific bioinformatics applications in the subsequent sections, underscoring the significance of this discipline in the pursuit of unraveling mesothelioma’s mysteries.
II. Bioinformatics for Early Diagnosis
The early diagnosis of mesothelioma is a critical component in improving patient outcomes. Bioinformatics plays a pivotal role in this endeavor, leveraging omics data to discover biomarkers, identify transcriptomics gene signatures, and analyze microRNA profiles for the early detection of mesothelioma.
1. Biomarker Discovery through Omics Data Analysis:
- Genomic Biomarkers: Bioinformatics analyzes genomic data to identify specific genetic alterations and mutations that serve as potential biomarkers for mesothelioma.
- Proteomic Signatures: By scrutinizing proteomic data, bioinformatics aids in the discovery of protein biomarkers indicative of early-stage mesothelioma.
- Metabolomic Fingerprints: Examining metabolomic profiles allows for the identification of unique metabolic signatures associated with early mesothelioma, contributing to biomarker discovery.
2. Transcriptomics Gene Signatures for Diagnosis:
- Differential Gene Expression Analysis: Bioinformatics techniques enable the identification of differentially expressed genes between normal and mesothelioma tissues, leading to the development of transcriptomic gene signatures.
- Machine Learning Approaches: Machine learning algorithms applied to transcriptomic data enhance the accuracy of distinguishing mesothelioma from benign conditions, aiding in early diagnosis.
- Functional Enrichment Analysis: Functional enrichment analysis helps interpret the biological relevance of identified gene signatures, shedding light on the underlying molecular processes in early-stage mesothelioma.
3. MicroRNA Profiles to Detect Tumors Earlier:
- MicroRNA Biomarkers: Bioinformatics explores microRNA profiles to uncover specific microRNAs that serve as biomarkers for early tumor detection.
- Diagnostic Models: Integrating microRNA expression patterns with computational models allows for the development of diagnostic tools capable of detecting mesothelioma at earlier stages.
- Circulating MicroRNAs: Analysis of circulating microRNAs in biofluids, facilitated by bioinformatics, offers a non-invasive approach for early detection and monitoring of mesothelioma.
Clinical Implications and Future Directions:
- Non-Invasive Diagnostic Tools: Biomarkers and gene signatures identified through bioinformatics pave the way for non-invasive diagnostic tools, such as blood tests or imaging techniques, facilitating early detection.
- Improved Screening Programs: The integration of bioinformatics-driven biomarkers into screening programs holds the potential to enhance the accuracy and efficiency of early mesothelioma detection.
- Patient Stratification: Early diagnosis, guided by bioinformatics, enables the stratification of patients based on their molecular profiles, allowing for more tailored and effective treatment strategies.
Challenges and Ongoing Research:
- Validation Studies: Ongoing research focuses on conducting rigorous validation studies to confirm the reliability and accuracy of bioinformatics-driven biomarkers in diverse patient populations.
- Dynamic Biomarker Panels: Identifying dynamic biomarker panels that evolve with disease progression remains a challenge, necessitating continuous investigation into evolving omics technologies.
Conclusion:
Bioinformatics serves as a beacon of hope in the quest for early diagnosis in mesothelioma. Through the discovery of biomarkers, analysis of transcriptomics gene signatures, and exploration of microRNA profiles, bioinformatics not only contributes to the early detection of mesothelioma but also holds the potential to transform screening and diagnostic paradigms. As research progresses, the integration of bioinformatics-driven approaches into clinical practice promises to usher in a new era where mesothelioma can be identified at its earliest, most treatable stages, ultimately improving patient outcomes.
III. Bioinformatics for Prognosis Prediction
Prognosis prediction is a critical aspect of mesothelioma management, guiding clinicians in tailoring treatment strategies. Bioinformatics plays a pivotal role in this realm by mapping genomic and transcriptomic patterns, identifying mutations linked to survival rates, and employing machine learning models for accurate prognosis in mesothelioma patients.
1. Mapping Genomic and Transcriptomic Patterns:
- Comprehensive Data Integration: Bioinformatics integrates genomic and transcriptomic data to map patterns that provide insights into the molecular landscape of mesothelioma.
- Identification of Altered Pathways: Analysis of altered pathways and molecular networks aids in understanding the biological processes influencing prognosis.
- Correlation with Clinical Outcomes: Mapping patterns allows for the correlation of genomic and transcriptomic alterations with clinical outcomes, forming the basis for prognostic indicators.
2. Identifying Mutations Linked to Survival Rates:
- Survival-Associated Mutations: Bioinformatics identifies mutations associated with differential survival rates in mesothelioma patients.
- Prognostic Biomarkers: Specific genomic alterations, discovered through bioinformatics analyses, may serve as prognostic biomarkers, offering valuable information about disease trajectory.
- Functional Consequences: Understanding the functional consequences of identified mutations provides insights into their impact on disease progression and patient outcomes.
3. Machine Learning Models for Prognosis:
- Feature Selection: Bioinformatics-driven machine learning models employ feature selection algorithms to identify the most relevant genomic and transcriptomic features for prognosis prediction.
- Risk Stratification: Machine learning models stratify patients into risk categories, providing nuanced predictions of survival outcomes based on their molecular profiles.
- Clinical Variables Integration: Integration with clinical variables enhances the predictive accuracy of machine learning models, considering a holistic set of factors influencing prognosis.
Clinical Implications and Future Directions:
- Tailored Treatment Planning: Prognostic information derived from bioinformatics analyses assists clinicians in tailoring treatment plans based on the predicted outcomes for individual mesothelioma patients.
- Stratified Clinical Trials: Prognostic models contribute to the stratification of patients in clinical trials, ensuring more homogeneous cohorts for evaluating treatment efficacy.
- Patient Counseling: Accurate prognosis prediction facilitates informed patient counseling, allowing individuals and their families to make decisions aligned with expected outcomes.
Challenges and Ongoing Research:
- Validation Across Datasets: Ongoing research focuses on validating bioinformatics-derived prognostic models across diverse datasets to ensure generalizability and reliability.
- Temporal Dynamics: Investigating the temporal dynamics of genomic and transcriptomic changes and their impact on prognosis is an evolving area of research.
Conclusion:
Bioinformatics empowers prognosis prediction in mesothelioma by deciphering intricate genomic and transcriptomic patterns. The identification of mutations linked to survival rates and the application of machine learning models refine our ability to predict patient outcomes with increasing precision. As bioinformatics methodologies advance and prognostic models continue to evolve, their integration into clinical decision-making holds the promise of enhancing the management of mesothelioma, guiding personalized treatment approaches, and ultimately improving patient prognoses.
IV. Bioinformatics Guiding Treatment
Bioinformatics serves as a guiding force in tailoring treatment strategies for mesothelioma, identifying targetable mutations and pathways, facilitating precision medicine approaches, and leveraging pharmacogenomics to make informed drug recommendations.
1. Finding Targetable Mutations and Pathways:
- Genomic Alterations: Bioinformatics analyzes genomic data to identify specific mutations that can be targeted with precision therapies.
- Pathway Analysis: Examining altered pathways in mesothelioma allows for the identification of key targets for intervention.
- Functional Annotation: Bioinformatics annotates the functional consequences of genomic alterations, aiding in the selection of targetable mutations with therapeutic relevance.
2. Bioinformatics for Precision Medicine:
- Patient Stratification: Bioinformatics facilitates the stratification of mesothelioma patients based on their molecular profiles, allowing for more precise and personalized treatment plans.
- Targeted Therapies: Precision medicine approaches guided by bioinformatics focus on the development and application of targeted therapies tailored to individual patients’ genomic and molecular characteristics.
- Adaptive Treatment Strategies: Continuous monitoring and bioinformatics-driven analysis enable adaptive treatment strategies, optimizing therapeutic efficacy based on evolving molecular profiles.
3. Pharmacogenomics and Drug Recommendations:
- Genetic Variability in Drug Response: Pharmacogenomics, guided by bioinformatics, explores how genetic variability influences individual responses to chemotherapy and other drugs.
- Drug-Gene Interactions: Bioinformatics assesses drug-gene interactions, identifying genetic factors that may predict drug efficacy, toxicity, or adverse reactions.
- Personalized Treatment Plans: By integrating pharmacogenomic information, bioinformatics contributes to the development of personalized treatment plans, optimizing drug selection and dosages for individual patients.
Clinical Implications and Future Directions:
- Improved Treatment Outcomes: Bioinformatics-guided treatment strategies aim to improve treatment outcomes by targeting specific genomic alterations and tailoring interventions to the unique molecular characteristics of each patient.
- Reduced Adverse Effects: Precision medicine, informed by bioinformatics, minimizes the risk of adverse effects by selecting treatments based on individual patient profiles.
- Enhanced Response Rates: Targeting specific mutations and pathways identified through bioinformatics enhances the likelihood of treatment responses, particularly in patients with actionable genomic alterations.
Challenges and Ongoing Research:
- Resistance Mechanisms: Ongoing research investigates the underlying genomic and molecular mechanisms of treatment resistance in mesothelioma, informing the development of strategies to overcome resistance.
- Clinical Implementation: Translating bioinformatics-driven treatment recommendations into routine clinical practice poses challenges related to validation, standardization, and integration into existing healthcare workflows.
Conclusion:
Bioinformatics is a linchpin in the era of precision medicine for mesothelioma, guiding treatment decisions based on the unique genomic and molecular landscape of each patient. By identifying targetable mutations and pathways, facilitating personalized treatment approaches, and leveraging pharmacogenomics for informed drug recommendations, bioinformatics contributes to optimizing therapeutic strategies. As research advances, the seamless integration of bioinformatics into clinical workflows holds the potential to revolutionize mesothelioma treatment, offering more effective and tailored interventions for individuals facing this challenging cancer.
V. Biobanks and Data Sharing
The establishment of biobanks and collaborative data-sharing initiatives has become instrumental in advancing mesothelioma research. This section explores prominent databases such as CaMic and TCGAMeso Hub, emphasizing the critical role of standardized data and integrated platforms in fostering a collaborative and data-rich landscape.
1. Databases like CaMic and TCGAMeso Hub:
- CaMic (Cancer Microenvironment database):
- Comprehensive Data Repository: CaMic serves as a comprehensive repository, collecting data related to the tumor microenvironment in mesothelioma.
- Multi-Omics Integration: The database integrates multi-omics data, including genomics, transcriptomics, and proteomics, providing a holistic view of mesothelioma biology.
- Research Collaboration: CaMic facilitates collaboration among researchers by offering a centralized platform for accessing and sharing data related to the mesothelioma microenvironment.
- TCGAMeso Hub (The Cancer Genome Atlas Mesothelioma Hub):
- Genomic Insights: TCGAMeso Hub focuses on genomic data specific to mesothelioma, offering insights into the genetic alterations and molecular characteristics of the disease.
- Large-Scale Collaborative Effort: The hub represents a collaborative effort, bringing together data generated by The Cancer Genome Atlas (TCGA) project, fostering a collective understanding of mesothelioma genetics.
- Accessible Resource: TCGAMeso Hub provides an accessible resource for researchers to explore, analyze, and integrate genomic information relevant to mesothelioma.
2. Importance of Data Standards and Integration:
- Standardized Data Formats: The adoption of standardized data formats ensures consistency across datasets, facilitating seamless integration and interoperability.
- Harmonization of Data: Data harmonization efforts, guided by standardized protocols, enable the integration of diverse datasets from different sources, creating a unified and comprehensive resource.
- Cross-Disciplinary Collaboration: Data integration across disciplines, such as genomics, transcriptomics, and clinical data, supports cross-disciplinary collaboration, allowing researchers to explore mesothelioma from multiple perspectives.
Clinical Implications and Future Directions:
- Enhanced Research Collaboration: Biobanks and data-sharing platforms enhance collaboration by providing a centralized space for researchers to contribute, access, and analyze mesothelioma-related data.
- Accelerated Discoveries: Access to large-scale, integrated datasets accelerates discoveries by offering a broader context for understanding mesothelioma biology and identifying potential therapeutic targets.
- Improved Patient Stratification: Integrated data from diverse sources contribute to improved patient stratification, guiding personalized treatment strategies based on a more comprehensive understanding of mesothelioma heterogeneity.
Challenges and Ongoing Research:
- Data Security and Privacy: Ensuring the security and privacy of patient data remains a challenge, requiring ongoing research to develop robust frameworks for ethical data sharing.
- Interoperability of Platforms: Efforts to enhance the interoperability of different data-sharing platforms and biobanks continue to be a focus of ongoing research.
Conclusion:
Biobanks and collaborative data-sharing initiatives play a pivotal role in advancing mesothelioma research by providing researchers with access to large, diverse datasets. Databases like CaMic and TCGAMeso Hub serve as valuable resources, offering insights into the complex biology of mesothelioma. The importance of data standards and integration cannot be overstated, as these factors are central to harnessing the full potential of collaborative efforts and accelerating discoveries in the field. As research progresses, the continued expansion and integration of biobanks and data-sharing platforms hold the promise of driving transformative breakthroughs in our understanding and treatment of mesothelioma.
VI. Future Outlook
The future of mesothelioma research holds exciting prospects, driven by the expansion of bioinformatics infrastructure, the training of proficient bioinformatics teams, and the accelerated pace of biomarker discovery facilitated by artificial intelligence (AI). This section delves into the potential advancements and strategies that will shape the landscape of mesothelioma research in the coming years.
1. Expanding Bioinformatics Infrastructure:
- Integration of Multi-Omics Data: Future initiatives will focus on enhancing the integration of diverse omics data, including genomics, transcriptomics, proteomics, and metabolomics, to provide a comprehensive understanding of mesothelioma.
- Cloud-Based Platforms: The adoption of cloud-based bioinformatics platforms will likely expand, offering scalable and accessible solutions for the storage, analysis, and sharing of large-scale genomic datasets.
- Real-Time Analytics: Advancements in real-time analytics will enable researchers to perform on-the-fly analyses, allowing for dynamic insights into the evolving molecular landscape of mesothelioma.
2. Training Bioinformatics Teams:
- Interdisciplinary Training: Future efforts will prioritize interdisciplinary training programs, equipping bioinformatics teams with a diverse skill set encompassing biology, computer science, and statistics.
- Collaborative Skillsets: Bioinformatics teams will be trained to collaborate effectively with clinicians, molecular biologists, and data scientists, fostering a synergistic approach to mesothelioma research.
- Continuous Professional Development: Ongoing education and continuous professional development will be emphasized to keep bioinformatics teams abreast of evolving technologies and methodologies.
3. Accelerating Biomarker Discovery with AI:
- Machine Learning and Deep Learning Algorithms: AI, particularly machine learning and deep learning algorithms, will play a pivotal role in accelerating biomarker discovery. These algorithms can analyze vast datasets, identify patterns, and predict potential biomarkers with enhanced accuracy.
- Integration of Multi-Modal Data: AI-driven approaches will facilitate the integration of multi-modal data, allowing for a more comprehensive exploration of the molecular and clinical landscape of mesothelioma.
- Automated Pipeline Development: The development of automated bioinformatics pipelines, guided by AI, will streamline the analysis of high-throughput data, expediting the identification of novel biomarkers.
Clinical Implications and Future Directions:
- Precision Medicine Implementation: The advancements in bioinformatics infrastructure and biomarker discovery driven by AI are poised to usher in an era of precision medicine for mesothelioma, where treatment strategies are tailored to individual patient profiles.
- Data-Driven Clinical Trials: Bioinformatics, coupled with AI, will inform the design of data-driven clinical trials, optimizing patient selection, and improving the efficiency of therapeutic interventions.
- Patient-Centric Approaches: Future research will prioritize patient-centric approaches, utilizing bioinformatics insights to enhance early detection, prognosis prediction, and treatment personalization.
Challenges and Ongoing Research:
- Ethical Data Use: Addressing ethical considerations related to data use and privacy will be an ongoing area of research, ensuring that advancements in bioinformatics align with ethical standards.
- Interoperability and Standardization: Efforts to improve the interoperability and standardization of bioinformatics tools and datasets will continue, promoting seamless collaboration and data integration.
Conclusion:
The future outlook for mesothelioma research is characterized by the integration of advanced bioinformatics infrastructure, the cultivation of skilled interdisciplinary teams, and the transformative impact of AI on biomarker discovery. These advancements hold the promise of not only deepening our understanding of mesothelioma but also revolutionizing the way we diagnose and treat this complex cancer. As bioinformatics continues to evolve, its central role in unraveling the mysteries of mesothelioma is poised to lead us toward more effective and personalized approaches for patients affected by this challenging disease.