Mesothelioma: Unraveling the Deadly Mysteries Through Bioinformatics and Genomics
November 28, 2023Table of Contents
I. Introduction
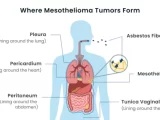
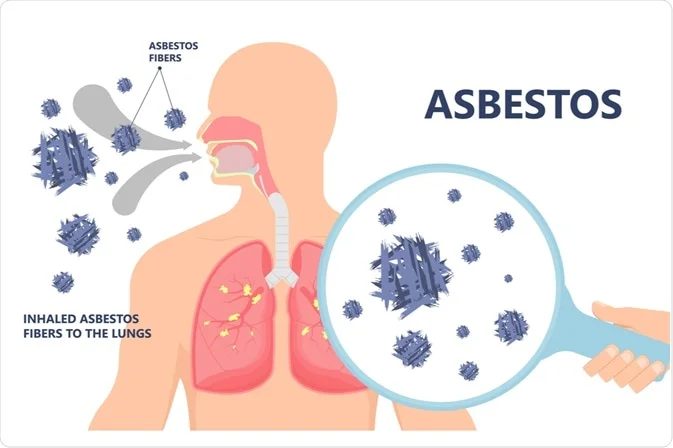
Mesothelioma, a deadly cancer associated with asbestos exposure, presents a complex and intriguing puzzle for medical researchers. Asbestos, a naturally occurring mineral once widely used in various industries, has been linked to the development of mesothelioma, a malignancy that primarily affects the protective linings of the lungs, abdomen, or heart. Despite advancements in medical science, significant mysteries surround mesothelioma, particularly in understanding why only some individuals exposed to asbestos develop this aggressive form of cancer.
Brief Background on Mesothelioma:
Mesothelioma arises due to the inhalation or ingestion of asbestos fibers, which can lead to cellular damage and the development of cancerous cells. Its latency period, spanning several decades, further complicates early detection and intervention. With symptoms often manifesting in the advanced stages, mesothelioma poses significant challenges for effective treatment.
The Mysteries Surrounding Mesothelioma:
- Variable Susceptibility: The puzzling variability in susceptibility to mesothelioma among individuals with asbestos exposure raises questions about genetic and environmental factors influencing disease development. Some exposed individuals remain unaffected, while others succumb to this aggressive cancer.
- Molecular Mechanisms: The precise molecular mechanisms underlying the transformation of asbestos-exposed cells into cancerous cells remain elusive. Understanding these mechanisms is essential for targeted therapies and prevention strategies.
- Asbestos Exposure Duration: The correlation between the duration of asbestos exposure and the likelihood of developing mesothelioma is not fully elucidated. Gaps persist in understanding the threshold levels and cumulative effects leading to disease manifestation.
Bioinformatics and Genomics in Unraveling Mysteries:
The emergence of bioinformatics and genomics has opened new avenues for unraveling the mysteries of mesothelioma. These cutting-edge technologies enable researchers to analyze vast amounts of genomic data, shedding light on the intricate genetic and molecular landscape associated with asbestos-induced carcinogenesis.
- Genomic Profiling: Through genomic profiling, researchers can identify genetic alterations, mutations, and expression patterns specific to mesothelioma. This information offers insights into the underlying biological processes and potential therapeutic targets.
- Biomarker Discovery: Bioinformatics facilitates the discovery of mesothelioma-specific biomarkers, aiding in early detection and personalized treatment approaches. Biomarkers can also help distinguish between asbestos-exposed individuals who develop mesothelioma and those who remain unaffected.
- Pathway Analysis: Genomic data analysis allows for the comprehensive exploration of signaling pathways and molecular networks implicated in mesothelioma. This knowledge contributes to a deeper understanding of disease progression and potential intervention points.
In conclusion, the mysteries surrounding mesothelioma provide a compelling incentive for leveraging bioinformatics and genomics. By dissecting the genomic intricacies associated with asbestos exposure and mesothelioma development, researchers aim to unlock crucial insights that can inform prevention strategies, enhance early diagnosis, and pave the way for targeted therapies in the battle against this devastating cancer.
II. Genetic Factors That Increase Mesothelioma Risk
Mesothelioma, a complex and aggressive cancer primarily linked to asbestos exposure, exhibits a multifaceted etiology that includes genetic factors contributing to an individual’s susceptibility to the disease. Among the genetic elements associated with an increased risk of mesothelioma, BRCA1 mutations and the BAP1 genetic mutation stand out prominently, shedding light on the intricate interplay between genetic predisposition and environmental carcinogens.
1. BRCA1 Mutations and Mesothelioma Predisposition:
- Background: BRCA1, a well-known tumor suppressor gene associated with breast and ovarian cancers, has been implicated in mesothelioma susceptibility.
- Link to Mesothelioma: Research suggests that individuals harboring BRCA1 mutations may be predisposed to mesothelioma, potentially due to the gene’s role in DNA repair mechanisms.
2. BAP1 Genetic Mutation and Tumor Growth:
- Background: BAP1 (BRCA1-associated protein 1) is a crucial gene involved in chromatin remodeling and tumor suppression.
- Strong Link to Mesothelioma: The BAP1 gene is strongly linked to mesothelioma, with studies indicating that mutations in BAP1 are associated with increased tumor growth and progression in mesothelioma cases.
- Prognostic Marker: BAP1 mutations have also been identified as prognostic markers, influencing the overall survival and clinical outcomes of mesothelioma patients.
3. Exploration of Other Genomic Variants:
- Current Research: Ongoing research is actively investigating additional genomic variants that may contribute to the risk of developing mesothelioma.
- Polygenic Risk Factors: The identification of polygenic risk factors, involving multiple genes and genetic variations, is a focus area in understanding the genetic basis of mesothelioma susceptibility.
Potential Implications and Future Directions:
- Precision Medicine Approaches: Knowledge of specific genetic factors associated with mesothelioma risk allows for the development of precision medicine approaches. Tailoring interventions based on an individual’s genetic profile could enhance treatment efficacy.
- Early Detection and Screening: Genetic markers associated with increased mesothelioma risk may serve as valuable tools for early detection and screening efforts, particularly among individuals with a history of asbestos exposure.
- Therapeutic Targets: Understanding the genetic underpinnings of mesothelioma opens avenues for identifying therapeutic targets. Targeted therapies can be developed to counteract the specific molecular alterations associated with mesothelioma predisposition and progression.
In conclusion, the identification of genetic factors such as BRCA1 mutations and BAP1 genetic alterations provides crucial insights into the genetic landscape of mesothelioma. Ongoing research into other genomic variants holds promise for unraveling additional risk factors and refining our understanding of the intricate interplay between genetics and environmental exposures in the development of this challenging cancer. These findings not only deepen our comprehension of mesothelioma pathogenesis but also offer potential avenues for improved risk assessment, early detection, and targeted therapeutic strategies.
III. Bioinformatics Approaches to Classify Mesothelioma Subtypes
Mesothelioma, characterized by its heterogeneity, presents a challenge in classification due to variations in clinical behavior and treatment response. Bioinformatics plays a pivotal role in unraveling the complexities of mesothelioma subtypes, utilizing transcriptomics, epigenomics, and proteomics to elucidate distinct molecular profiles and enhance our understanding of the disease for more tailored therapeutic interventions.
1. Transcriptomics Analysis for Gene Expression Patterns:
- Objective: Transcriptomics focuses on studying the complete set of RNA transcripts to understand gene expression patterns.
- Approach: By analyzing transcriptomic data, researchers can identify specific gene expression signatures associated with different mesothelioma subtypes.
- Classification Basis: Clustering based on gene expression profiles enables the classification of mesothelioma into subtypes, providing insights into the underlying biology and potential therapeutic targets.
2. Epigenomics Studies to Unravel Activation Mechanisms:
- Objective: Epigenomics explores modifications to DNA and associated proteins, shedding light on the regulatory mechanisms influencing gene activation and silencing.
- Approach: Understanding the epigenetic landscape of mesothelioma helps decipher why certain genes become activated, driving disease progression.
- Identification of Biomarkers: Epigenomic studies contribute to the identification of epigenetic biomarkers, which can aid in subtype classification and provide valuable information for targeted therapies.
3. Proteomics Utilizing Biomarkers for Cell Type Distinction:
- Objective: Proteomics involves the comprehensive study of proteins, offering insights into the functional aspects of cells.
- Approach: Proteomic analyses leverage biomarkers to distinguish mesothelioma cell types based on protein expression profiles.
- Clinical Implications: Identification of distinct protein signatures enables the development of diagnostic and prognostic tools, guiding treatment decisions and improving patient outcomes.
Integration of Multiple Omics Data:
- Holistic Understanding: The integration of transcriptomic, epigenomic, and proteomic data provides a more comprehensive understanding of mesothelioma subtypes.
- Precision Medicine Applications: A multi-omics approach facilitates the identification of molecularly defined subtypes, paving the way for more personalized and effective treatment strategies.
Clinical Significance and Future Directions:
- Improved Diagnosis and Prognosis: Bioinformatics-driven subtype classification enhances the accuracy of mesothelioma diagnosis and prognosis.
- Targeted Therapies: Molecular insights gained from these bioinformatics approaches guide the development of targeted therapies tailored to specific mesothelioma subtypes.
- Longitudinal Monitoring: Biomarkers identified through these analyses can be used for longitudinal monitoring of disease progression and treatment response.
In conclusion, the integration of bioinformatics approaches, encompassing transcriptomics, epigenomics, and proteomics, is instrumental in classifying mesothelioma subtypes. These comprehensive analyses not only contribute to our understanding of the molecular basis of the disease but also hold significant promise for advancing precision medicine applications, ultimately improving diagnosis, prognosis, and treatment strategies for individuals affected by mesothelioma.
IV. Early Detection Advances Using MicroRNA Profiles
Advances in early detection methods are critical for improving outcomes in mesothelioma, a cancer often diagnosed at advanced stages. MicroRNA profiling has emerged as a promising avenue, with the potential to enable the diagnosis of mesothelioma at earlier stages. The development of custom bioinformatic tools has further enhanced the ability to detect tumor-specific microRNA profiles in blood samples, presenting a significant stride towards improving early treatment strategies and ultimately boosting survival rates.
1. MicroRNA Patterns for Early Diagnosis:
- Rationale: MicroRNAs are small non-coding RNA molecules that play a crucial role in gene regulation. Aberrant expression of microRNAs is associated with various cancers, including mesothelioma.
- Early Detection Potential: Distinct microRNA expression patterns have been identified in mesothelioma, offering the potential for early detection before the onset of clinical symptoms.
2. Custom Bioinformatic Tools for Profiling:
- Bioinformatic Tools: The development of specialized bioinformatic tools facilitates the analysis of complex microRNA profiles in a high-throughput and customized manner.
- Tumor-Specific Signatures: These tools enable the identification of tumor-specific microRNA signatures, allowing for precise and reliable detection of mesothelioma.
3. Detection of Tumor MicroRNA Profiles in Blood:
- Non-Invasive Approach: Blood-based assays offer a non-invasive method for early detection by capturing circulating tumor-derived microRNAs.
- Liquid Biopsy Significance: Liquid biopsy, focusing on microRNA profiles in blood, provides a minimally invasive alternative to traditional tissue biopsies.
Clinical Goals and Implications:
- Improving Early Treatment Initiatives: Early detection through microRNA profiling creates opportunities for initiating treatment at more manageable stages, potentially improving therapeutic responses.
- Survival Rate Enhancement: The overarching goal is to enhance survival rates by diagnosing mesothelioma at a stage where interventions are more effective and the disease burden is lower.
Challenges and Future Directions:
- Standardization and Validation: Standardizing methodologies and validating the reliability of microRNA profiles for mesothelioma detection across diverse patient populations remain crucial.
- Integration with Imaging Techniques: Combining microRNA profiling with imaging techniques may offer a comprehensive approach for early diagnosis.
Broader Implications for Cancer Detection:
- Paradigm Shift: The integration of microRNA profiling into early detection strategies represents a broader paradigm shift in cancer diagnosis, emphasizing precision and minimally invasive approaches.
In conclusion, the utilization of microRNA profiles for early detection of mesothelioma, coupled with custom bioinformatic tools, marks a significant advancement in cancer research. This approach holds great promise in transforming the landscape of mesothelioma diagnosis, with the ultimate aim of improving early treatment outcomes and enhancing the survival rates of individuals affected by this challenging cancer. Continued research, validation, and integration with existing diagnostic modalities will be pivotal for realizing the full potential of microRNA profiling in the early detection of mesothelioma and other cancers.
V. Precision Medicine: Custom Treatments Through Genetics
Precision medicine, driven by insights from genomic research, has revolutionized the approach to cancer treatment. In the context of mesothelioma, the identification of specific genetic factors influencing tumor growth patterns has paved the way for tailoring treatment strategies at an individualized level. This includes the customization of chemotherapy drugs based on a patient’s unique genomic landscape and the development of advanced immunotherapy approaches targeting specific mesothelioma proteins.
1. Identifying Genetic Factors Affecting Tumor Growth:
- Genetic Profiling: Comprehensive genomic profiling enables the identification of specific genetic alterations driving mesothelioma tumor growth.
- Individual Variability: Recognizing the genetic diversity among mesothelioma patients informs the selection of targeted therapies based on the unique genetic factors contributing to each individual’s disease progression.
2. Tailoring Chemotherapy Drugs Based on Genomic Landscape:
- Personalized Chemotherapy: Precision medicine allows for the customization of chemotherapy regimens based on an individual’s genomic profile.
- Optimizing Drug Selection: Understanding the genetic makeup of mesothelioma tumors assists in selecting chemotherapy drugs with a higher likelihood of efficacy, minimizing adverse effects.
3. Immunotherapy Advances Targeting Mesothelioma Proteins:
- Immunotherapeutic Targets: Precision medicine facilitates the identification of specific mesothelioma proteins that can serve as immunotherapeutic targets.
- Personalized Immunotherapy: Tailoring immunotherapy approaches based on the unique protein expression patterns in a patient’s mesothelioma cells enhances treatment precision.
Clinical Benefits and Treatment Outcomes:
- Enhanced Treatment Efficacy: Precision medicine aims to improve treatment outcomes by matching interventions to the specific genetic characteristics of an individual’s mesothelioma.
- Reduced Side Effects: Customizing treatments based on genomic information may reduce the occurrence of side effects, as therapies are selected with consideration for individual variations in drug metabolism.
Challenges and Future Directions:
- Validation and Standardization: Ensuring the validation and standardization of precision medicine approaches for mesothelioma treatment across diverse patient populations remains a challenge.
- Integration with Multimodal Therapies: Combining precision medicine with other therapeutic modalities, such as surgery or radiation, requires careful coordination for optimal treatment outcomes.
Broader Implications for Cancer Treatment:
- Paradigm Shift in Oncology: Precision medicine represents a paradigm shift in cancer treatment, moving away from one-size-fits-all approaches to tailored interventions based on individual genetic profiles.
- Expanding Applications: The success of precision medicine in mesothelioma treatment holds implications for applying similar approaches to other cancers, further advancing the era of personalized oncology.
In conclusion, precision medicine in mesothelioma treatment, involving the identification of genetic factors influencing tumor growth and the customization of therapies based on individual genomic landscapes, marks a transformative stride in cancer care. The integration of advanced immunotherapy targeting specific mesothelioma proteins further enhances the precision and efficacy of treatment strategies. While challenges persist, the ongoing evolution of precision medicine holds significant promise for improving the prognosis and quality of life for individuals facing mesothelioma and sets the stage for a new era in cancer therapeutics.
VI. The Future of Mesothelioma Research
Mesothelioma research stands at the cusp of transformative developments, propelled by collaborative efforts, advanced technologies, and a growing understanding of the genomic and biotechnological landscape. The future of mesothelioma research holds great promise, driven by initiatives such as expanding biobanks, international data sharing efforts, increased funding for bioinformatics cancer research, and the optimistic pursuit of innovative and lifesaving approaches in genomics and biotechnology.
1. Expanding Biobanks and International Data Sharing:
- Global Collaboration: Collaborative efforts to expand biobanks and share data on an international scale are essential for aggregating diverse genomic information.
- Diversity in Data: A broader representation of patient populations in biobanks enhances the diversity of genomic data, providing a more comprehensive understanding of mesothelioma across different demographics.
2. Increased Funding for Bioinformatics Cancer Research:
- Resource Allocation: Adequate funding is crucial for advancing bioinformatics cancer research, enabling the development of sophisticated tools, algorithms, and methodologies.
- Multi-Omics Integration: Increased funding supports the integration of multi-omics approaches, allowing researchers to delve deeper into the complexities of mesothelioma at the genomic, epigenomic, and proteomic levels.
3. Hope for Discovering New Lifesaving Approaches:
- Genomics as a Catalyst: The future of mesothelioma research hinges on genomics as a catalyst for discovering novel therapeutic targets and precision medicine strategies.
- Biotechnological Advancements: Biotechnological innovations, including targeted therapies and immunotherapies, offer hope for groundbreaking approaches that may revolutionize mesothelioma treatment.
Clinical Translation and Patient Impact:
- Translation to Clinical Practice: The ultimate goal is to translate research findings into clinical practices, ensuring that advancements directly benefit patients diagnosed with mesothelioma.
- Improved Therapeutic Strategies: The future holds the promise of more effective and less invasive therapeutic strategies, resulting in improved patient outcomes and quality of life.
Challenges and Navigating the Future Landscape:
- Ethical Considerations: As research expands, ethical considerations regarding data privacy, consent, and the responsible use of genetic information become increasingly important.
- Interdisciplinary Collaboration: The interdisciplinary collaboration between bioinformaticians, clinicians, geneticists, and biotechnologists is essential for overcoming challenges and realizing the full potential of mesothelioma research.
Engaging the Public and Advocacy:
- Raising Awareness: Public engagement and advocacy efforts are crucial for raising awareness about mesothelioma, fostering support, and garnering the necessary resources for continued research.
- Patient-Centric Research: Ensuring that research is patient-centric, addressing the needs and perspectives of individuals affected by mesothelioma, is vital for meaningful progress.
In conclusion, the future of mesothelioma research is marked by a collective commitment to expanding knowledge, breaking down silos through international collaboration, securing funding for bioinformatics advancements, and harnessing the potential of genomics and biotechnology. With an optimistic outlook, researchers aspire to not only deepen our understanding of mesothelioma but also pave the way for transformative discoveries that hold the potential to reshape the landscape of diagnosis and treatment for this challenging cancer.