Decoding the Mesothelioma Genome: Unlocking New Therapeutic Horizons
November 28, 2023Table of Contents
I. Introduction
Mesothelioma, a malignancy primarily associated with asbestos exposure, stands as a formidable adversary in the realm of cancer, characterized by its deadly nature and limited treatment options. This introductory exploration delves into the profound challenges posed by mesothelioma, emphasizing the imperative of comprehending the genomic blueprint of tumors to unravel the mysteries surrounding its development and progression.
Deadly Nature of Mesothelioma and Treatment Challenges:
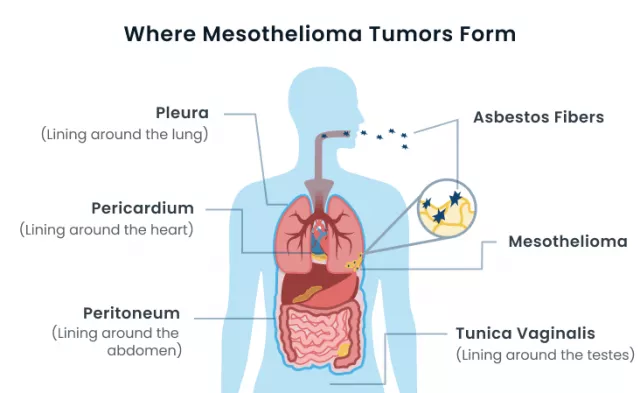
Mesothelioma, arising from the mesothelial cells lining the lungs, abdomen, or heart, is notorious for its aggressive nature and often eludes early detection. The latency period between asbestos exposure and symptom manifestation compounds the challenge, leading to advanced stages at diagnosis. Treatment options for mesothelioma are limited, and the prognosis is frequently unfavorable, necessitating a deeper understanding of the molecular underpinnings to pave the way for more effective interventions.
Understanding the Genomic Blueprint of Mesothelioma Tumors:
- Genomic Complexity:
- Mesothelioma exhibits a complex genomic landscape, with intricate alterations influencing tumor behavior.
- Unraveling the genomic blueprint involves deciphering genetic mutations, copy number variations, and epigenetic modifications driving mesothelioma progression.
- Importance of Genomic Insights:
- Understanding the genomic intricacies is pivotal for unraveling the mysteries surrounding why some individuals develop mesothelioma after asbestos exposure.
- Genomic insights inform the identification of potential therapeutic targets and the development of personalized treatment strategies.
- Challenges in Genomic Research:
- Genomic research in mesothelioma encounters challenges such as tumor heterogeneity and the scarcity of large-scale genomic datasets.
- Overcoming these challenges is crucial for obtaining a comprehensive view of the genomic blueprint and translating findings into meaningful clinical applications.
Research Goals and Future Prospects:
- Personalized Therapies: The ultimate goal is to pave the way for personalized therapies tailored to the unique genomic profiles of mesothelioma tumors.
- Early Detection Advances: Genomic insights may contribute to the development of early detection methods, offering hope for interventions at more manageable stages.
In the face of mesothelioma’s formidable challenges and limited treatment options, delving into the genomic blueprint of tumors emerges as a beacon of hope. This journey of understanding holds the potential to transform the landscape of mesothelioma research, offering insights that can guide innovative therapeutic approaches and, ultimately, improve the prognosis for individuals confronting this devastating cancer.
II. Sequencing Mesothelioma Genomes
Sequencing mesothelioma genomes represents a pivotal step in unraveling the intricate genetic landscape underlying this aggressive cancer. Employing advanced sequencing methods, such as whole genome and exome sequencing, and leveraging bioinformatics analyses, researchers aim to identify somatic mutations and chromosomal alterations. This comprehensive exploration delves into the methodologies and insights gained through sequencing mesothelioma genomes.
1. Whole Genome and Exome Sequencing Methods:
- Whole Genome Sequencing (WGS):
- Comprehensive Coverage: WGS involves sequencing the entire genome, providing a comprehensive view of both coding and non-coding regions.
- Identification of Variants: It allows for the identification of single nucleotide variations (SNVs), insertions, deletions, and structural variations across the entire genome.
- Exome Sequencing:
- Targeted Coding Regions: Exome sequencing focuses specifically on the protein-coding regions of the genome.
- Cost-Effective Approach: While capturing a smaller portion of the genome, exome sequencing is a cost-effective method for identifying variants within coding regions where most functional mutations occur.
2. Bioinformatics Analysis of Somatic Mutations:
- Somatic Mutation Identification:
- Distinguishing Germline and Somatic Mutations: Bioinformatics tools play a crucial role in distinguishing between germline and somatic mutations, highlighting alterations specific to mesothelioma tumors.
- Variant Calling: Algorithms are employed for variant calling, identifying genetic changes that accumulate during the development of cancer.
- Functional Annotation:
3. Identifying Chromosomal Alterations:
- Copy Number Variations (CNVs):
- Amplifications and Deletions: Sequencing data is analyzed to detect CNVs, revealing regions of the genome that are either amplified or deleted.
- Implications for Tumor Biology: Identification of CNVs provides insights into key genes and pathways implicated in mesothelioma biology.
- Structural Variations:
- Rearrangements and Fusions: Sequencing methods uncover structural variations, including chromosomal rearrangements and gene fusions.
- Cancer Driver Events: Detection of structural variations aids in identifying potential driver events contributing to mesothelioma development.
Clinical Implications and Future Directions:
- Biomarker Discovery:
- Personalized Treatment Targets: Sequencing mesothelioma genomes contributes to the discovery of potential biomarkers, paving the way for personalized treatment targets.
- Predictive and Prognostic Markers: Identifying somatic mutations and chromosomal alterations may yield predictive and prognostic markers guiding therapeutic decisions.
- Therapeutic Opportunities:
- Targeted Therapies: Insights gained from sequencing efforts may lead to the identification of molecular targets for developing targeted therapies.
- Immunotherapeutic Strategies: Understanding the genomic landscape aids in exploring immunotherapeutic strategies tailored to mesothelioma’s unique genetic characteristics.
Sequencing mesothelioma genomes through advanced methods and bioinformatics analyses unveils the genetic complexities of this cancer. The identification of somatic mutations and chromosomal alterations not only enhances our understanding of mesothelioma biology but also opens avenues for innovative therapeutic strategies, bringing us closer to more effective and personalized approaches for combating this formidable disease.
III. Key Genomic Mutations Driving Mesothelioma
Mesothelioma, characterized by its aggressive nature, is driven by specific genomic mutations that underlie its development and progression. Key mutations in genes such as BAP1, NF2, and CDKN2A play pivotal roles in mesothelioma pathogenesis. This exploration delves into these genomic alterations, the disrupted biological pathways in mesothelioma, and the genomic heterogeneity observed among patients.
1. BAP1 (BRCA1-Associated Protein 1) Mutations:
- Role in Mesothelioma: BAP1 is a tumor suppressor gene, and mutations in BAP1 are strongly associated with mesothelioma.
- Tumor Suppression Mechanism: BAP1 is involved in chromatin remodeling and DNA repair, and its loss of function contributes to uncontrolled cell growth.
- Prognostic Significance: BAP1 mutations have prognostic implications, influencing the clinical outcomes and overall survival of mesothelioma patients.
2. NF2 (Neurofibromatosis Type 2) Mutations:
- Involvement in Mesothelioma: NF2 is a tumor suppressor gene, and its mutations are implicated in the development of mesothelioma.
- Regulation of Cell Growth: NF2 encodes a protein that regulates cell growth by suppressing signaling pathways. Mutations lead to the loss of this regulatory function, contributing to mesothelioma progression.
- Association with Benign Mesothelioma: NF2 mutations are also associated with benign mesothelioma, emphasizing its role in the mesothelial cell environment.
3. CDKN2A (Cyclin-Dependent Kinase Inhibitor 2A) Mutations:
- Function and Impact: CDKN2A is a crucial gene that regulates the cell cycle by inhibiting cyclin-dependent kinases.
- Tumor Suppression: Mutations in CDKN2A result in the loss of its tumor-suppressive function, contributing to uncontrolled cell division.
- Frequency in Mesothelioma: CDKN2A mutations are frequently observed in mesothelioma cases, further underscoring their significance in the disease.
4. Disrupted Biological Pathways in Mesothelioma:
- Cell Cycle Dysregulation: Mutations in genes like CDKN2A contribute to dysregulation of the cell cycle, allowing uncontrolled cell division.
- Apoptosis Evasion: Alterations in apoptotic pathways contribute to the evasion of programmed cell death, a hallmark of cancer cells.
- DNA Repair Mechanisms: Mutations in genes involved in DNA repair, such as BAP1, compromise the cell’s ability to correct genetic errors, fostering genomic instability.
5. Genomic Heterogeneity Between Patients:
- Inter-Individual Variation: Mesothelioma exhibits significant genomic heterogeneity, with variations in the mutational landscape between individual patients.
- Clinical Implications: Genomic heterogeneity poses challenges in identifying universal therapeutic targets, emphasizing the need for personalized treatment strategies.
- Intra-Tumor Heterogeneity: Intra-tumor heterogeneity further complicates treatment approaches, as different regions within a single tumor may harbor distinct genomic alterations.
Clinical and Therapeutic Implications:
- Targeted Therapies: Understanding key genomic mutations informs the development of targeted therapies directed at specific molecular vulnerabilities.
- Prognostic Markers: Certain mutations, such as those in BAP1, serve as prognostic markers, guiding treatment decisions and predicting patient outcomes.
Conclusion:
Key genomic mutations, including those in BAP1, NF2, and CDKN2A, drive the complex landscape of mesothelioma. The disrupted biological pathways and genomic heterogeneity observed in mesothelioma patients underscore the challenges and opportunities in understanding the molecular basis of this aggressive cancer. This knowledge serves as a foundation for developing targeted therapies and advancing precision medicine approaches in the quest to improve outcomes for individuals affected by mesothelioma.
IV. Targeting Mutations for Novel Therapies
Harnessing the understanding of specific mutations driving mesothelioma, novel therapeutic strategies are emerging to target these genomic alterations. This exploration focuses on innovative approaches in clinical trials, aiming to exploit synthetic lethality for BAP1 mutations, employing focal adhesion kinase (FAK) inhibitors for NF2 mutations, and leveraging checkpoint immunotherapy in the context of CDKN2A mechanisms.
1. Clinical Trials for BAP1 Synthetic Lethality:
- Rationale: BAP1 mutations in mesothelioma create vulnerabilities that can be exploited through synthetic lethality.
- Poly (ADP-ribose) Polymerase (PARP) Inhibitors: Clinical trials are investigating the use of PARP inhibitors, which exploit the impaired DNA repair mechanisms in BAP1-deficient cells, leading to cell death.
- Personalized Treatment: Targeting synthetic lethality offers a personalized therapeutic approach based on the specific genomic characteristics of mesothelioma tumors with BAP1 mutations.
2. NF2 Mutation Strategies with Focal Adhesion Kinase (FAK) Inhibitors:
- Role of NF2 in Mesothelioma: NF2 mutations contribute to mesothelioma development and progression.
- Focal Adhesion Kinase (FAK) Inhibitors: Clinical investigations are exploring FAK inhibitors as a targeted therapeutic strategy for mesothelioma with NF2 mutations.
- Disruption of FAK Signaling: Inhibiting FAK disrupts signaling pathways implicated in cell adhesion and migration, potentially impeding the growth of NF2-mutant mesothelioma cells.
3. CDKN2A Mechanisms and Checkpoint Immunotherapy:
- CDKN2A Mutations in Mesothelioma: Mesothelioma frequently harbors mutations in the CDKN2A gene, leading to dysregulated cell cycle control.
- Checkpoint Immunotherapy: Strategies involving checkpoint immunotherapy, such as PD-1/PD-L1 inhibitors, aim to enhance the immune system’s ability to recognize and target cancer cells.
- Overcoming Immune Evasion: CDKN2A mutations may contribute to immune evasion, and checkpoint inhibitors seek to overcome this by restoring anti-tumor immune responses.
Clinical and Therapeutic Outlook:
- Precision Medicine Paradigm: Targeting specific mutations aligns with the precision medicine paradigm, tailoring therapies based on the unique genomic characteristics of individual mesothelioma cases.
- Improved Treatment Efficacy: Novel therapies addressing BAP1 synthetic lethality, NF2-mutant pathways, and CDKN2A-related immune evasion have the potential to enhance treatment efficacy and outcomes.
Challenges and Future Directions:
- Resistance Mechanisms: Understanding and overcoming potential resistance mechanisms to these targeted therapies is a critical area of ongoing research.
- Combination Therapies: Investigating the efficacy of combining targeted therapies with standard treatments or other emerging approaches holds promise for enhanced therapeutic benefits.
In the pursuit of novel therapies for mesothelioma, targeting specific genomic mutations, such as BAP1, NF2, and CDKN2A, represents a frontier of innovation. Clinical trials exploring synthetic lethality, FAK inhibitors, and checkpoint immunotherapy exemplify the commitment to advancing precision medicine and improving outcomes for individuals affected by mesothelioma. As research progresses, the potential for more effective, personalized, and targeted therapeutic approaches offers hope in the ongoing battle against this challenging cancer.
V. Overcoming Resistance in Precision Medicine
While precision medicine holds promise, overcoming resistance is a critical challenge in the treatment of mesothelioma. This section explores strategies to address resistance through RNA profiling of resistant tumors, the identification of new combinatorial drug targets, and the advent of adaptive therapy protocols.
1. RNA Profiling of Resistant Tumors:
- Understanding Resistance Mechanisms: RNA profiling of tumors that develop resistance to targeted therapies provides insights into the molecular mechanisms underlying resistance.
- Identification of Altered Pathways: Transcriptomic analysis helps identify changes in gene expression and signaling pathways that contribute to the development of resistance.
- Personalized Adaptations: Understanding the specific RNA profiles of resistant tumors facilitates the development of personalized strategies to overcome resistance.
2. Identifying New Combinatorial Drug Targets:
- Comprehensive Genomic Analysis: Integrating genomic and transcriptomic data enables a comprehensive analysis of the molecular landscape of resistant tumors.
- Exploration of Combinatorial Targets: Identification of new molecular targets, either within the same pathway or in parallel pathways, allows the development of combinatorial drug approaches.
- Synergistic Therapies: Combining drugs that target distinct aspects of the resistant phenotype may overcome resistance and enhance treatment efficacy.
3. Advent of Adaptive Therapy Protocols:
- Dynamic Treatment Adjustments: Adaptive therapy involves dynamically adjusting treatment regimens based on the evolving characteristics of the tumor.
- Optimizing Treatment Response: By continuously monitoring tumor responses and adapting therapies accordingly, adaptive protocols aim to optimize treatment efficacy and overcome resistance.
- Minimizing Selection Pressure: Adaptive therapy seeks to reduce the selective pressure on resistant subpopulations, potentially slowing down or preventing the emergence of resistance.
Clinical Implications and Future Directions:
- Individualized Treatment Plans: Strategies to overcome resistance aim to individualize treatment plans, tailoring interventions based on the specific molecular features and adaptive changes observed in each patient.
- Integration with Clinical Trials: Developing and implementing these resistance-overcoming strategies often involves integration with ongoing clinical trials, ensuring a rigorous and evidence-based approach.
Challenges and Ongoing Research:
- Tumor Heterogeneity: The heterogeneous nature of mesothelioma tumors poses challenges in identifying universal strategies to overcome resistance.
- Longitudinal Monitoring: Longitudinal monitoring of patients and their tumors is crucial for adapting therapies in real-time and understanding the dynamics of resistance evolution.
As precision medicine continues to advance, addressing resistance becomes paramount for improving treatment outcomes in mesothelioma. Leveraging RNA profiling, identifying new combinatorial targets, and implementing adaptive therapy protocols represent dynamic approaches to navigate and overcome the challenges posed by resistance. Ongoing research endeavors in these areas hold the potential to usher in a new era of more effective and personalized therapeutic strategies for individuals facing this formidable cancer.
VI. The Future of Genomics-Driven Research
The future of genomics-driven research holds immense promise, driven by initiatives such as expanding open-source genomic databases, the pursuit of curative treatments through genomics, and the optimistic outlook for mesothelioma patients entering a new era of precision medicine.
1. Expanding Open-Source Genomic Databases:
- Global Collaboration: The expansion of open-source genomic databases fosters global collaboration, allowing researchers worldwide to access and contribute to a vast repository of genomic data.
- Diverse Patient Populations: Inclusivity in data collection enhances the representation of diverse patient populations, contributing to a more comprehensive understanding of mesothelioma across different demographics.
- Accelerating Research: Open-source databases serve as accelerators for research, providing a foundation for the development and validation of novel findings and therapeutic approaches.
2. Towards Curative Treatments Through Genomics:
- Precision Therapies: Genomics-driven research is steering the development of precision therapies, targeting specific genetic alterations implicated in mesothelioma.
- Curative Intent: The ultimate goal is to shift towards curative treatments, leveraging insights from genomics to eradicate mesothelioma at its roots.
- Multifaceted Approaches: Integrating genomics with other ‘omics’ disciplines and advanced technologies contributes to a multifaceted approach aimed at achieving curative outcomes.
3. Hope for Mesothelioma Patients in the New Era of Precision Medicine:
- Tailored Treatment Approaches: Mesothelioma patients stand to benefit from the personalized and tailored treatment approaches made possible by precision medicine.
- Early Detection and Intervention: Genomics-driven strategies hold promise for early detection and intervention, potentially transforming the prognosis for individuals diagnosed with mesothelioma.
- Improved Quality of Life: Precision medicine not only targets the cancer but also seeks to improve the overall quality of life for patients by minimizing treatment-related side effects through targeted interventions.
Clinical Integration and Patient Impact:
- Clinical Translation: Bridging the gap between genomics research and clinical applications is critical for translating promising findings into tangible benefits for patients.
- Patient-Centric Approaches: Ensuring that genomics-driven research remains patient-centric involves addressing the specific needs and perspectives of individuals affected by mesothelioma.
Challenges and Collaborative Solutions:
- Ethical Considerations: As genomics research advances, addressing ethical considerations, including data privacy and consent, remains crucial.
- Interdisciplinary Collaboration: The collaboration between genomics researchers, clinicians, bioinformaticians, and other experts is essential for overcoming challenges and realizing the full potential of precision medicine in mesothelioma.
Conclusion:
The future of genomics-driven research in mesothelioma is marked by collaborative endeavors, a commitment to open science, and the anticipation of transformative outcomes. As open-source genomic databases expand, curative treatments become a tangible goal, and precision medicine enters a new era, there is hope for mesothelioma patients to witness groundbreaking advancements that redefine the landscape of diagnosis, treatment, and ultimately, the prognosis for this challenging cancer.