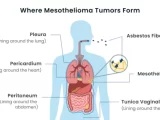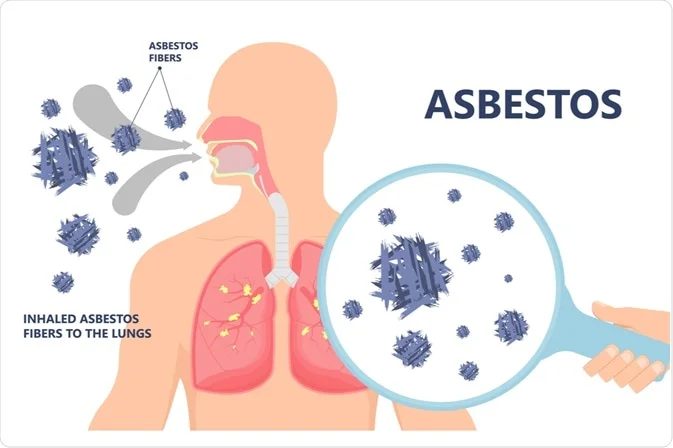
Mesothelioma: A Bioinformatics and Genomics Odyssey
November 28, 2023Table of Contents
I. Introduction
The landscape of mesothelioma research is undergoing a profound transformation with the advent of the molecular era. This introduction sets the stage for an exploration into how molecular insights, driven by advancements in genomics, transcriptomics, proteomics, and other molecular technologies, are reshaping our understanding of mesothelioma. As we delve into this molecular era, the complexities of mesothelioma’s biology are being unraveled, offering new avenues for early detection, precision medicine, and innovative therapeutic approaches.
1. Unveiling the Molecular Complexity:
- Genomic Landscape: Advances in genomic technologies have unveiled the intricate genomic landscape of mesothelioma, highlighting specific mutations, chromosomal alterations, and pathways driving the disease.
- Transcriptomic Signatures: Transcriptomics studies have provided insights into gene expression patterns, revealing unique signatures that distinguish mesothelioma subtypes and offer clues to its underlying biology.
- Proteomic Profiling: Proteomic analyses have elucidated the protein-level alterations in mesothelioma, shedding light on functional changes and potential therapeutic targets.
2. The Promise of Precision Medicine:
- Personalized Treatment Strategies: The molecular era opens doors to precision medicine, where treatment strategies are tailored to the individual molecular profile of each mesothelioma patient.
- Biomarker Discovery: Molecular insights drive biomarker discovery, identifying specific markers that can aid in early diagnosis, prognosis prediction, and monitoring treatment responses.
- Targeted Therapies: The molecular understanding of mesothelioma paves the way for targeted therapies, aiming to intervene in specific molecular pathways responsible for tumor growth and progression.
3. Challenges and Opportunities:
- Tumor Heterogeneity: The molecular era confronts the challenge of tumor heterogeneity within mesothelioma, prompting researchers to unravel the diverse molecular profiles that exist across different patients and even within the same tumor.
- Data Integration: Integrating diverse molecular data types, including genomics, transcriptomics, and proteomics, poses both a challenge and an opportunity to comprehensively understand the complexity of mesothelioma.
4. Collaborative Research Initiatives:
- Biobanks and Data Sharing: Collaborative initiatives, such as biobanks and data-sharing platforms, become essential in the molecular era, facilitating the pooling of large-scale molecular datasets for comprehensive analyses.
- Interdisciplinary Teams: The interdisciplinary collaboration of researchers, clinicians, bioinformaticians, and data scientists becomes imperative to navigate the complexities of molecular data and translate findings into clinical applications.
Conclusion:
As mesothelioma research enters the molecular era, the exploration of its genomic, transcriptomic, and proteomic landscape holds immense promise. The molecular insights gained not only deepen our understanding of the disease but also pave the way for transformative approaches in diagnosis and treatment. This journey into the molecular intricacies of mesothelioma heralds a new era of precision and personalized medicine, where advancements driven by molecular technologies aim to improve outcomes for individuals affected by this challenging cancer.
II. Charting the Cancer Genome
The advent of high-throughput sequencing methods has revolutionized our ability to decipher the intricate genomic landscape of mesothelioma. This section explores the significance of high-throughput sequencing technologies in charting the cancer genome, leading to the discovery of key genomic alterations that underlie the complexities of mesothelioma.
1. High-Throughput Sequencing Methods:
- Next-Generation Sequencing (NGS):
- Parallel Sequencing: NGS enables parallel sequencing of millions of DNA fragments, offering unprecedented speed and efficiency in genomic analysis.
- Whole Genome Sequencing (WGS): WGS provides a comprehensive view of the entire genome, uncovering both coding and non-coding regions with high resolution.
- Targeted Sequencing (Panel Sequencing): Targeted sequencing focuses on specific genomic regions, allowing for a more in-depth analysis of genes associated with mesothelioma.
- RNA Sequencing (RNA-Seq):
- Transcriptomic Insights: RNA-Seq provides insights into gene expression patterns, alternative splicing, and non-coding RNA molecules, contributing to our understanding of mesothelioma at the transcriptomic level.
- Single-Cell Sequencing:
- Unraveling Heterogeneity: Single-cell sequencing techniques enable the exploration of intra-tumor heterogeneity, revealing diverse molecular profiles within individual mesothelioma tumors.
2. Discovery of Mesothelioma Genomic Landscape:
- Identification of Driver Mutations:
- BAP1 Mutations: High-throughput sequencing has identified BAP1 mutations as key driver mutations in mesothelioma, providing insights into the molecular mechanisms underlying tumor development.
- NF2 and CDKN2A Alterations: Genomic studies have uncovered alterations in NF2 and CDKN2A, contributing to the dysregulation of cellular processes and highlighting potential therapeutic targets.
- Chromosomal Aberrations:
- Copy Number Variations (CNVs): High-throughput methods reveal CNVs, such as deletions in tumor suppressor genes and amplifications in oncogenes, offering a comprehensive view of chromosomal aberrations in mesothelioma.
- Chromosomal Rearrangements: Structural variations, including chromosomal rearrangements, are unveiled, providing insights into the genomic instability characteristic of mesothelioma.
- Epigenomic Discoveries:
- DNA Methylation Patterns: High-throughput sequencing of DNA methylation patterns elucidates epigenetic changes associated with mesothelioma, impacting gene expression and contributing to disease progression.
Clinical Implications and Future Directions:
- Personalized Treatment Approaches: Genomic discoveries inform the development of personalized treatment approaches, targeting specific mutations and pathways identified through high-throughput sequencing.
- Biomarker Identification: The genomic landscape uncovered by high-throughput methods serves as a rich source for biomarker discovery, with potential applications in early diagnosis, prognosis prediction, and treatment monitoring.
- Advancements in Functional Genomics: Integrating high-throughput genomic data with functional genomics approaches advances our understanding of how genomic alterations drive mesothelioma at the molecular and cellular levels.
Challenges and Ongoing Research:
- Tumor Heterogeneity Challenges: Ongoing research focuses on addressing challenges posed by tumor heterogeneity, leveraging advanced sequencing technologies to capture the diverse molecular profiles within mesothelioma tumors.
- Integrating Multi-Omics Data: Efforts continue to integrate data from multiple omics layers, such as genomics, transcriptomics, and epigenomics, to create a comprehensive molecular atlas of mesothelioma.
Conclusion:
High-throughput sequencing methods have been instrumental in charting the cancer genome of mesothelioma, unraveling its genomic complexity. The discoveries made through these technologies provide a foundation for understanding the molecular basis of mesothelioma, paving the way for innovative therapeutic strategies and personalized approaches to patient care. As sequencing technologies continue to evolve, the molecular insights gained will drive advancements in precision medicine and contribute to ongoing efforts to improve outcomes for individuals affected by mesothelioma.
III. Voyage Through the Transcriptome
Embarking on a journey through the transcriptome of mesothelioma, this section delves into the RNA profiling of mesothelioma cells and pathways. By mapping dynamic gene expression patterns, researchers gain a deeper understanding of the molecular intricacies that govern mesothelioma progression and heterogeneity.
1. RNA Profiling of Mesothelioma Cells and Pathways:
- Transcriptomics Technologies:
- RNA Sequencing (RNA-Seq): High-throughput RNA-Seq technologies capture the transcriptome of mesothelioma cells, providing a comprehensive snapshot of gene expression.
- Microarray Analysis: Microarray technology, although older, remains valuable for profiling gene expression patterns in mesothelioma, offering insights into the dynamics of RNA abundance.
- Identification of Differentially Expressed Genes:
- Tumor vs. Normal Comparison: Transcriptomic analyses compare the gene expression profiles of mesothelioma tumors with normal tissues, revealing genes that are differentially expressed and potentially implicated in tumorigenesis.
- Subtype-Specific Signatures: Researchers identify subtype-specific gene expression signatures, unraveling the molecular distinctions between various mesothelioma subtypes.
2. Mapping Dynamic Gene Expression Patterns:
- Temporal Expression Changes:
- Longitudinal Studies: Longitudinal transcriptomic studies uncover dynamic changes in gene expression over time, shedding light on the temporal evolution of mesothelioma and its response to treatments.
- Time-Series Analyses: Time-series analyses capture the dynamic nature of gene expression, offering insights into how gene regulatory networks evolve during mesothelioma progression.
- Functional Enrichment Analysis:
- Pathway Analysis: Functional enrichment analysis identifies biological pathways that are enriched or dysregulated in mesothelioma, providing a systems-level understanding of the disease.
- Gene Set Enrichment Analysis (GSEA): GSEA elucidates the coordinated regulation of gene sets, offering a nuanced view of the transcriptomic landscape.
3. Clinical Implications and Future Directions:
- Biomarker Discovery:
- Diagnostic Biomarkers: Transcriptomic profiling contributes to the discovery of diagnostic biomarkers that distinguish mesothelioma from non-malignant conditions, facilitating early detection.
- Prognostic Signatures: Identification of prognostic gene expression signatures aids in predicting patient outcomes, guiding treatment decisions based on the molecular characteristics of individual tumors.
- Targeted Therapies:
- Precision Medicine Approaches: Transcriptomic insights guide precision medicine approaches, identifying specific genes and pathways as targets for tailored therapeutic interventions.
- Immunotherapy Targets: Mapping the transcriptome unveils potential immunotherapy targets, allowing for the development of immunotherapeutic strategies tailored to the molecular profile of mesothelioma.
Challenges and Ongoing Research:
- Single-Cell Transcriptomics: Ongoing research explores single-cell transcriptomics to dissect heterogeneity within mesothelioma tumors at the cellular level, revealing cell-specific expression profiles.
- Integration with Other Omics Data: Efforts continue to integrate transcriptomic data with genomic, proteomic, and epigenomic data to create a comprehensive multi-omics understanding of mesothelioma.
Conclusion:
The journey through the transcriptome of mesothelioma is a crucial expedition, unraveling the dynamic gene expression patterns that govern the behavior of this complex cancer. Transcriptomic insights not only contribute to biomarker discovery and the development of targeted therapies but also offer a roadmap for understanding the temporal dynamics of mesothelioma progression. As transcriptomics technologies evolve and become more sophisticated, the ongoing exploration of the mesothelioma transcriptome holds the promise of deeper insights into its biology and the development of innovative therapeutic strategies.
IV. Exploring the Proteome Universe
Embarking on an exploration of the proteome universe in mesothelioma, this section delves into how mass spectrometry identifies protein drivers and the significance of global proteogenomic initiatives. Unraveling the intricacies of the proteome provides valuable insights into the functional aspects of mesothelioma, paving the way for a comprehensive understanding of its molecular landscape.
1. Mass Spectrometry Identifies Protein Drivers:
- Comprehensive Protein Analysis:
- Protein Identification: Mass spectrometry enables the identification of proteins present in mesothelioma cells, uncovering the diversity of the proteome.
- Quantitative Proteomics: Quantitative proteomic approaches assess changes in protein abundance, shedding light on the dysregulation of specific proteins in mesothelioma.
- Identification of Protein Drivers:
- Oncogenic Proteins: Mass spectrometry identifies oncoproteins and tumor suppressors that play pivotal roles in driving mesothelioma progression.
- Post-Translational Modifications (PTMs): Detection of PTMs provides insights into the regulatory mechanisms that modulate protein functions in mesothelioma.
2. Global Proteogenomic Initiatives:
- Integrating Proteomics with Genomics:
- Comprehensive Molecular Understanding: Global proteogenomic initiatives integrate proteomic data with genomic information, providing a holistic understanding of the molecular intricacies of mesothelioma.
- Correlation with Genomic Alterations: Proteogenomic analyses correlate protein expression patterns with genomic alterations, elucidating the functional consequences of genetic changes.
- Identification of Novel Targets:
- Targeted Therapies: Proteogenomic studies identify novel therapeutic targets by revealing proteins that are overexpressed, mutated, or exhibit aberrant post-translational modifications in mesothelioma.
- Drug Response Biomarkers: Understanding the proteogenomic landscape aids in the discovery of biomarkers that predict responses to specific drugs, guiding personalized treatment strategies.
3. Clinical Implications and Future Directions:
- Therapeutic Target Discovery:
- Precision Medicine Applications: Proteomic insights contribute to the discovery of therapeutic targets, supporting the development of precision medicine approaches for mesothelioma.
- Immunotherapy Targets: Identification of immunogenic proteins guides the development of immunotherapies, leveraging the immune system to target mesothelioma cells.
- Biomarker Discovery:
- Prognostic Biomarkers: Proteomic analyses contribute to the identification of prognostic biomarkers, aiding in the prediction of disease outcomes and guiding treatment decisions.
- Minimally Invasive Diagnostics: Protein biomarkers discovered through proteomic studies may be harnessed for minimally invasive diagnostic methods, such as blood-based tests.
Challenges and Ongoing Research:
- Single-Cell Proteomics: Ongoing research explores single-cell proteomics to capture heterogeneity within mesothelioma tumors at the protein level, revealing cell-specific protein expression profiles.
- Integration with Other Omics Data: Efforts continue to integrate proteomic data with genomic, transcriptomic, and epigenomic data to create a comprehensive multi-omics understanding of mesothelioma.
Conclusion:
Exploring the proteome universe in mesothelioma through mass spectrometry and global proteogenomic initiatives is a transformative endeavor. The identification of protein drivers and the integration of proteomic data with genomics offer unparalleled insights into the functional aspects of mesothelioma. These insights not only advance our understanding of the disease at the molecular level but also hold significant promise for the development of targeted therapies, personalized medicine, and innovative diagnostic approaches. As proteomics technologies continue to evolve, the ongoing exploration of the mesothelioma proteome is poised to play a pivotal role in shaping the future of precision oncology.
V. Navigation by Biomarkers
Navigating the complex landscape of mesothelioma involves leveraging bioinformatics to pinpoint biomarkers. This section explores how bioinformatics plays a crucial role in the quest for diagnostic and prognostic signatures, offering valuable tools for personalized medicine and enhancing clinical decision-making.
1. Leveraging Bioinformatics to Pinpoint Markers:
- Integration of Multi-Omics Data:
- Genomic Data Integration: Bioinformatics integrates genomic data to identify genetic markers associated with mesothelioma, unraveling key mutations and variations.
- Transcriptomic Insights: Leveraging transcriptomic data, bioinformatics uncovers gene expression markers that distinguish mesothelioma subtypes and provide insights into disease progression.
- Proteomic Signatures: Bioinformatics analyzes proteomic data to identify protein markers associated with mesothelioma, contributing to the discovery of therapeutic targets.
- Machine Learning Algorithms:
- Pattern Recognition: Bioinformatics employs machine learning algorithms to recognize patterns in omics data, facilitating the identification of novel biomarkers with high accuracy.
- Feature Selection: Machine learning aids in selecting relevant features from large datasets, streamlining the identification of key biomarkers that characterize mesothelioma.
2. Quest for Diagnostic and Prognostic Signatures:
- Diagnostic Biomarkers:
- Early Detection Strategies: Bioinformatics-driven analyses contribute to the identification of diagnostic biomarkers, enabling early detection of mesothelioma when treatment options may be more effective.
- Minimally Invasive Testing: Biomarkers identified through bioinformatics may be explored for minimally invasive diagnostic tests, such as blood-based assays.
- Prognostic Indicators:
- Survival Predictors: Bioinformatics tools analyze molecular data to discover prognostic biomarkers that predict patient survival outcomes, aiding in treatment planning and risk stratification.
- Treatment Response Markers: Identification of biomarkers associated with treatment response guides personalized therapeutic approaches, enhancing the precision of mesothelioma management.
3. Clinical Implications and Future Directions:
- Personalized Treatment Strategies:
- Targeted Therapies: Biomarkers identified through bioinformatics guide the development of targeted therapies, tailoring treatment strategies based on the specific molecular characteristics of individual patients.
- Immunotherapy Selection: Bioinformatics facilitates the identification of immunotherapy-responsive biomarkers, informing the selection of patients likely to benefit from immunotherapeutic interventions.
- Dynamic Biomarker Panels:
- Dynamic Monitoring: Ongoing research focuses on dynamic biomarker panels that can be monitored over time, providing real-time insights into disease progression and treatment response.
- Adaptive Treatment Strategies: Biomarker-driven adaptive treatment strategies leverage bioinformatics to adjust therapies based on evolving molecular profiles, optimizing therapeutic efficacy.
Challenges and Ongoing Research:
- Validation and Standardization: Ongoing research addresses challenges related to the validation and standardization of biomarkers, ensuring their reliability and reproducibility in diverse clinical settings.
- Dynamic Biomarker Identification: Advancements in bioinformatics aim to capture the dynamic nature of mesothelioma by identifying biomarkers that evolve over the course of the disease.
Conclusion:
The navigation by biomarkers, guided by bioinformatics, is instrumental in transforming mesothelioma research and clinical practice. From diagnostic markers enabling early detection to prognostic indicators informing treatment decisions, bioinformatics plays a central role in unraveling the molecular intricacies of mesothelioma. As research continues, the integration of multi-omics data and the development of dynamic biomarker panels hold the promise of ushering in a new era of precision medicine, where personalized treatment strategies are refined through a deep understanding of the molecular landscape of mesothelioma.
VI. At the Dawn of Precision Health
As we stand at the dawn of precision health, this section explores how bioinformatics is instrumental in targeting genomic mutations and pathways, guiding therapeutic choices in the pursuit of personalized and effective treatments for mesothelioma.
1. Targeting Genomic Mutations and Pathways:
- Precision Genomic Medicine:
- Identification of Driver Mutations: Bioinformatics analyzes genomic data to pinpoint driver mutations in mesothelioma, guiding the development of targeted therapies.
- Genomic Subtyping: Subtyping based on genomic alterations allows for a nuanced understanding of mesothelioma, enabling tailored treatments for specific molecular subgroups.
- Pathway Analysis:
- Dysregulated Pathways: Bioinformatics-driven pathway analysis reveals dysregulated cellular pathways in mesothelioma, offering insights into potential vulnerabilities that can be targeted.
- Synthetic Lethality Approaches: Identifying synthetic lethal interactions guides the exploration of novel therapeutic strategies, exploiting specific vulnerabilities in mesothelioma cells.
2. Bioinformatics Guiding Therapeutic Choices:
- Predictive Biomarkers:
- Treatment Response Prediction: Bioinformatics identifies predictive biomarkers, allowing for the anticipation of how individual patients may respond to specific treatments.
- Pharmacogenomics Insights: Understanding the pharmacogenomics of mesothelioma through bioinformatics informs drug selection, optimizing treatment regimens based on individual genomic profiles.
- Immunotherapy Strategies:
- Immunogenic Biomarkers: Bioinformatics uncovers immunogenic biomarkers in mesothelioma, guiding the selection of patients likely to benefit from immunotherapies.
- Tumor Microenvironment Analysis: Assessing the tumor microenvironment through bioinformatics aids in predicting responses to immunotherapeutic interventions.
3. Clinical Implementation and Future Directions:
- Individualized Treatment Plans:
- Tailored Therapeutic Approaches: The integration of bioinformatics into clinical practice allows for the development of individualized treatment plans, optimizing the chances of therapeutic success.
- Adaptive Treatment Strategies: Bioinformatics-driven approaches pave the way for adaptive treatment strategies, adjusting therapies based on evolving genomic landscapes and treatment responses.
- Real-Time Decision Support:
- Dynamic Clinical Decision-Making: Bioinformatics tools provide real-time decision support, assisting clinicians in making dynamic treatment decisions informed by the latest molecular data.
- Clinical Trials Matching: Bioinformatics facilitates the identification of suitable clinical trials, matching patients with mesothelioma to novel therapies based on their genomic profiles.
Challenges and Ongoing Research:
- Data Integration Challenges: Ongoing research addresses challenges related to the integration of diverse omics data, ensuring a comprehensive and accurate representation of the molecular landscape of mesothelioma.
- Validation and Standardization: Efforts continue to validate and standardize bioinformatics approaches for clinical use, ensuring their reliability and reproducibility.
Conclusion:
At the dawn of precision health, bioinformatics emerges as a key driver in the quest for effective and personalized treatments for mesothelioma. By targeting genomic mutations and pathways, bioinformatics refines our understanding of the disease’s molecular complexity, paving the way for tailored therapeutic choices. As bioinformatics applications in clinical settings continue to evolve, the vision of precision health becomes increasingly tangible, offering hope for improved outcomes and quality of life for individuals affected by mesothelioma.
VII. The Odyssey Continues
As the odyssey through mesothelioma research traverses uncharted dimensions, this section emphasizes the collaborative efforts in exploring new frontiers together. The ultimate goal remains steadfast — the continuous pursuit of improving patient outcomes through the integration of cutting-edge technologies, interdisciplinary collaboration, and a commitment to unraveling the complexities of mesothelioma.
1. Traversing Uncharted Dimensions Together:
- Interdisciplinary Collaboration:
- Team Science Approach: Researchers, clinicians, bioinformaticians, and data scientists collaborate in a team science approach, combining expertise to navigate the complexities of mesothelioma.
- Data Integration Across Disciplines: Uncharted dimensions are explored by integrating data from genomics, transcriptomics, proteomics, and other disciplines, creating a comprehensive understanding of the disease.
- Technological Innovations:
- Emerging Technologies: Embracing emerging technologies, such as single-cell sequencing, spatial transcriptomics, and advanced imaging techniques, opens new frontiers in mesothelioma research.
- Artificial Intelligence Applications: Integrating artificial intelligence enhances the analysis of vast datasets, uncovering patterns and insights that may remain elusive through traditional methods.
2. Ultimate Goal of Improving Patient Outcomes:
- Patient-Centric Approaches:
- Tailored Therapies: The odyssey seeks to translate molecular insights into tailored therapies, ensuring that treatment approaches are precisely matched to the individual characteristics of each patient’s mesothelioma.
- Minimally Invasive Monitoring: Continuous advancements aim to develop minimally invasive methods for monitoring disease progression and treatment responses, prioritizing patient comfort and well-being.
- Global Collaboration:
- Biobank Initiatives: The establishment and expansion of biobank initiatives, coupled with international data-sharing efforts, create a global collaborative network to accelerate mesothelioma research.
- Pooling Expertise: Researchers worldwide pool their expertise, share insights, and collectively contribute to the advancement of knowledge, fostering a global community dedicated to improving patient outcomes.
3. Challenges and Future Endeavors:
- Tackling Heterogeneity: The odyssey faces the challenge of comprehensively addressing tumor heterogeneity, requiring ongoing research to capture the diverse molecular profiles within and between mesothelioma tumors.
- Ethical Considerations: As research progresses, ethical considerations regarding data privacy, consent, and responsible use of genomic information continue to be critical focal points.
Conclusion:
The odyssey through mesothelioma research continues as a collaborative journey, where researchers, clinicians, and scientists traverse uncharted dimensions together. The ultimate goal remains unwavering — to improve patient outcomes through a deep and nuanced understanding of mesothelioma’s molecular intricacies. As the odyssey unfolds, innovations in technology, interdisciplinary collaboration, and a commitment to patient-centric approaches propel the field forward. The collective efforts of the global research community contribute to the hope of transforming mesothelioma from a challenging diagnosis to a condition that can be effectively managed, if not cured.