Introduction to post-translational modifications
Introduction to Post-Translational Modifications (PTMs)
Definition and Significance of PTMs:
Post-Translational Modifications (PTMs) refer to the covalent and generally enzymatic modification of proteins following their synthesis. That is, after a protein is translated from a messenger RNA (mRNA) molecule during the process of protein synthesis, it can undergo various modifications. These modifications can change the physical and chemical properties of the protein, affecting its function, location, and interactions with other molecules.
PTMs play a critical role in the cell’s control over protein activity, localization, turnover, and interactions. They also provide a dynamic mechanism by which cells can rapidly alter protein functions in response to environmental cues or cellular signals.
Role in Expanding Protein Diversity:
- Diversification of Proteins: A single gene can give rise to multiple protein variants through different PTMs. This means that the actual diversity of the proteome (all the proteins expressed by an organism) can be much larger than its genome.
- Protein-Protein Interactions: PTMs can introduce new interaction motifs or remove existing ones, leading to the formation of different protein complexes.
- Protein Stability: Modifications like ubiquitination can tag a protein for degradation, affecting its stability and turnover in the cell.
- Functional Alteration: Some PTMs can change the activity of proteins. For instance, phosphorylation can activate or inhibit enzyme activity.
Importance in Regulating Protein Function:
- Signal Transduction: Many cellular signals are mediated through the phosphorylation of proteins. Kinases (enzymes that add phosphate groups) and phosphatases (enzymes that remove phosphate groups) play pivotal roles in these pathways.
- Subcellular Localization: PTMs can influence where in the cell a protein functions. For example, the addition of a lipid group might anchor a protein to a membrane.
- Protein Activity Modulation: As mentioned earlier, PTMs like phosphorylation can turn an enzyme’s activity on or off. Another example is the acetylation of histone proteins, which can control gene expression.
- Protein Degradation: Some PTMs target proteins for degradation, ensuring that they function only for a limited time or under specific conditions.
- Cell Cycle Control: PTMs control the progression of the cell cycle, ensuring that events like DNA replication and cell division occur in the correct order and only once per cycle.
In conclusion, PTMs are crucial for the functionality of proteins in the cellular environment. They introduce a dynamic level of control that enables the cell to respond rapidly to changes and challenges, thereby ensuring its survival and proper function.
Basics of Protein Synthesis and Structure
1. Brief Overview of DNA Transcription and RNA Translation:
DNA Transcription: It’s the process by which an RNA molecule, specifically messenger RNA (mRNA), is synthesized from a DNA template. In this process:
- DNA unwinds and unzips.
- RNA polymerase binds to a specific DNA sequence called a promoter.
- Using one strand of the DNA as a template, RNA polymerase synthesizes a complementary mRNA strand.
- Once transcription is complete, the mRNA molecule detaches from the DNA and moves out of the nucleus (in eukaryotic cells) and into the cytoplasm.
RNA Translation: This process transforms the mRNA sequence into a protein. Here’s how:
- The mRNA attaches to a ribosome, which reads its sequence three nucleotides at a time. Each set of three nucleotides, called a codon, corresponds to a specific amino acid.
- Transfer RNA (tRNA) molecules, which have an amino acid attached to one end and an anticodon on the other end, bind to the mRNA codons. The anticodon of the tRNA is complementary to the mRNA codon.
- As each tRNA binds, the ribosome helps form a bond between the adjacent amino acids, building the protein chain.
- This continues until a stop codon is reached, signaling the end of protein synthesis.
2. The Primary Structure of Proteins: Amino Acid Sequences
The primary structure of a protein refers to the linear sequence of amino acids in the polypeptide chain. This sequence determines the overall function and properties of the protein. A protein’s primary structure is dictated by the sequence of nucleotides in the mRNA, which in turn is transcribed from DNA.
3. Higher Order Protein Structures: Secondary, Tertiary, and Quaternary
Secondary Structure: This involves local folding of the polypeptide chain into specific shapes driven by hydrogen bonding between the backbone atoms of the amino acids. The most common secondary structures are α-helices and β-pleated sheets.
Tertiary Structure: This refers to the three-dimensional shape of the entire polypeptide chain, which results from interactions between the side chains (or R groups) of the amino acids. Types of interactions include hydrogen bonds, ionic bonds, disulfide bridges, and hydrophobic interactions. The tertiary structure gives a protein its functionality.
Quaternary Structure: Some proteins consist of multiple polypeptide chains (subunits) that function together as one protein complex. The arrangement and interaction of these subunits constitute the quaternary structure. Hemoglobin, with its four polypeptide subunits, is a classic example of a protein with quaternary structure.
To sum up, the process of protein synthesis begins with the transcription of DNA into mRNA, which is then translated to form a polypeptide chain. This chain undergoes various levels of folding—from primary to quaternary—to achieve its functional form. Each level of structure plays a critical role in determining the protein’s function and properties.
Common Types of Post-Translational Modifications (PTMs)
a. Phosphorylation
Mechanism and Role: Phosphorylation involves the addition of a phosphate group to specific amino acids in a protein, most commonly serine, threonine, and tyrosine residues. This modification often changes the activity, stability, or subcellular localization of the protein.
Kinases and Phosphatases: Enzymes Involved
- Kinases: Enzymes that catalyze the addition of phosphate groups to proteins.
- Phosphatases: Enzymes that remove phosphate groups, thereby reversing the action of kinases.
b. Acetylation
Mechanism and Role: Acetylation involves the addition of an acetyl group to lysine residues in proteins, affecting their activity, stability, or interactions.
Histone Acetylation and Gene Regulation: When histones (proteins around which DNA wraps) are acetylated, it generally leads to a relaxed chromatin structure, facilitating transcription and therefore promoting gene expression. Conversely, deacetylation leads to chromatin condensation and transcriptional repression.
c. Ubiquitination
Mechanism and Role: Ubiquitination involves the addition of ubiquitin, a small regulatory protein, to lysine residues in target proteins. This modification can lead to various outcomes, including protein degradation, cellular signaling, and DNA repair.
Proteasome and Protein Degradation: Polyubiquitinated proteins are often targeted for degradation by the 26S proteasome, a large protein complex responsible for degrading unnecessary or damaged proteins.
d. Methylation
Mechanism and Role: Methylation involves the addition of a methyl group to various residues in proteins, most commonly lysine and arginine residues. This can affect protein activity, interactions, or localization.
Histone Methylation and Gene Regulation: Methylation of histones can lead to either transcriptional activation or repression, depending on the specific lysine or arginine residue being methylated.
e. Glycosylation
O-linked and N-linked Glycosylation:
- O-linked Glycosylation: Involves the attachment of a sugar molecule to the oxygen atom of serine or threonine residues.
- N-linked Glycosylation: Involves the attachment of a sugar molecule to the nitrogen atom of an asparagine residue.
Importance in Protein Folding and Cell Signaling: Glycosylation plays a crucial role in protein folding, stability, and function. It also plays a vital role in cell signaling, especially in cell-to-cell recognition and adhesion.
f. Other PTMs
- Sulfation: Addition of a sulfate group, usually to tyrosine residues, playing roles in protein-protein interactions and cell signaling.
- Sumoylation: Addition of the SUMO (Small Ubiquitin-like Modifier) protein, affecting protein stability, activity, and localization.
- Nitrosylation: Addition of a nitric oxide group, which can affect protein activity and play roles in cellular signaling, especially in response to oxidative stress.
Brief Overview of Each: Each of these modifications provides an additional layer of regulation, allowing the cell to fine-tune protein function in response to various signals or environmental cues.
In summary, PTMs are vital for the regulation and diversification of protein function. They introduce dynamic and reversible changes to proteins, enabling cells to adapt and respond to their ever-changing environments swiftly.
Methods for Detecting and Analyzing PTMs
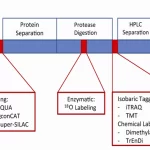
a. Mass Spectrometry (MS)
Introduction to MS and its Application to PTMs: Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles. In proteomics, MS can be used to identify proteins based on their unique mass and charge properties. When studying PTMs, MS can detect specific modifications because each PTM imparts a characteristic change in the mass of a protein or peptide.
Tandem MS for PTM Site Identification: Tandem mass spectrometry (often referred to as MS/MS) involves two stages of mass analysis. In the first stage, peptides with a particular mass are selected, and in the second stage, these selected peptides are fragmented to produce a series of smaller ions. The resulting patterns can be used to deduce the sequence of the peptide and to identify the exact site of modification.
b. Western Blotting
Western blotting is a method used to detect specific proteins in a sample. By using antibodies that recognize specific PTMs, one can detect and quantify the level of a particular modification.
Use of Antibodies Specific to Modified Residues: Antibodies can be generated to recognize specific modifications, such as phospho-serine or acetyl-lysine. By using these antibodies in a western blot, one can detect proteins that carry these modifications.
c. Proximity Ligation Assay
This is an in-situ method that allows detection and quantification of protein interactions, modifications, and protein expressions. It involves the use of two antibodies, each attached to a unique oligonucleotide. If the two antibodies are in close proximity due to binding to a target protein (or modification), the oligonucleotides can be joined and amplified, allowing for detection.
d. Chromatin Immunoprecipitation (ChIP)
Especially for Histone Modifications: ChIP is a technique used to determine the location of DNA binding sites on the genome for a particular protein of interest. For studying histone modifications, antibodies specific to modified histones are used to pull down the histone-DNA complexes. Once isolated, the associated DNA can be sequenced or analyzed to determine which genes or regions of the genome are associated with specific histone modifications.
e. Phospho-protein Specific Staining
Certain dyes or stains, such as Pro-Q Diamond, can be used to detect phospho-proteins specifically. These stains can be applied to gels after electrophoresis, allowing visualization of phosphorylated proteins directly.
In conclusion, the study of PTMs is crucial for understanding protein function and regulation. Multiple advanced techniques, like those mentioned above, offer researchers the tools to investigate and characterize these modifications in depth, shedding light on cellular processes and potential therapeutic targets.
Role of PTMs in Diseases and Disorders
PTMs in Cancer:
Alterations in PTMs can play significant roles in cancer onset, progression, and therapeutic resistance.
- Hyper-phosphorylation: A hallmark of many cancers is the dysregulation of kinases, leading to hyper-phosphorylation of numerous proteins. This can activate oncogenes or inactivate tumor suppressors. For example, the aberrant activation of the EGFR kinase or mutations in the BRAF kinase can lead to inappropriate cell signaling and cancer.
- Histone Modifications: Changes in histone acetylation and methylation can lead to abnormal gene expression patterns in cancer. Overexpression or mutation of enzymes that add or remove these modifications can drive oncogenesis.
Neurodegenerative Diseases:
PTMs play a significant role in various neurodegenerative conditions.
- Tau Phosphorylation in Alzheimer’s: In Alzheimer’s disease, the tau protein becomes abnormally phosphorylated. Hyper-phosphorylated tau forms aggregates known as neurofibrillary tangles, one of the pathological hallmarks of the disease. These tangles interfere with neuronal function and contribute to neurodegeneration.
Diabetes:
Insulin signaling is pivotal in glucose homeostasis, and PTMs play a role in this signaling pathway.
- Insulin Signaling and Phosphorylation: Insulin binding to its receptor activates a cascade of phosphorylation events, leading to glucose uptake. In insulin-resistant states, like type 2 diabetes, this signaling pathway can be impaired, often due to altered phosphorylation patterns or levels.
Immunological Diseases:
- Glycosylation and Autoimmune Responses: Changes in protein glycosylation patterns can sometimes be recognized as “foreign” by the immune system, leading to autoimmune responses. For instance, alterations in IgG glycosylation patterns have been implicated in the pathogenesis of rheumatoid arthritis.
Targeting PTMs for Therapeutic Interventions:
Given the pivotal roles of PTMs in various diseases, they present attractive targets for therapeutic interventions.
- Kinase Inhibitors: Many anticancer drugs, like imatinib (Gleevec) and vemurafenib, are kinase inhibitors. They target the dysregulated kinases in cancer cells, reducing hyper-phosphorylation events and inhibiting tumor growth.
- HDAC Inhibitors: Histone deacetylase (HDAC) inhibitors can change gene expression patterns in cancer cells, inducing cell cycle arrest, differentiation, or apoptosis.
- Tau Aggregation Inhibitors: For neurodegenerative diseases like Alzheimer’s, compounds that prevent tau aggregation or promote its clearance are under investigation.
- Glycosylation Modifiers: In diseases where aberrant glycosylation plays a role, enzymes involved in the glycosylation pathway can be targeted to restore normal patterns.
In conclusion, PTMs have a deep connection with various diseases, from their onset to progression. Understanding these modifications offers insights into disease mechanisms and provides avenues for targeted therapeutic interventions.
Post-Translational Modifications in Drug Development
PTMs as Drug Targets:
PTMs, by their nature, dynamically modulate protein function, activity, stability, and interactions. Because of this, they are intricately linked to various pathological processes when dysregulated. Thus, enzymes or pathways involved in adding or removing these modifications represent potential drug targets. By influencing the PTM status of proteins, therapeutics can rectify aberrant cellular signaling, restore physiological function, or induce desired cellular outcomes (like apoptosis in cancer cells).
The Challenge of Targeting Enzymes Responsible for PTMs:
- Specificity: Many enzymes that regulate PTMs, especially kinases, belong to large families with highly conserved active sites. Designing drugs that target one specific kinase or enzyme without affecting others can be challenging.
- Resistance: Just as with many targeted therapies, resistance can emerge. In the context of kinases, secondary mutations can arise that prevent the drug from binding while still allowing the kinase to function.
- Network Complexity: Proteins often do not operate in isolation but as part of complex networks. Altering the activity of one enzyme can have cascading effects, some of which might be counterproductive or harmful.
- Dynamic Nature of PTMs: Since PTMs are reversible and dynamic, maintaining a therapeutic effect might require continuous treatment, which can have implications for drug dosing and toxicity.
Success Stories: Kinase Inhibitors in Cancer Therapy:
- Imatinib (Gleevec): This drug revolutionized the treatment of chronic myeloid leukemia (CML). It targets the BCR-ABL fusion kinase, a product of the Philadelphia chromosome and a driver of CML. Imatinib binds to the kinase’s active site, preventing its action and leading to cancer cell death.
- Vemurafenib: Designed for melanoma patients with a specific BRAF mutation (V600E), this drug inhibits the mutant BRAF kinase. This halts the overactive signaling pathway driven by the mutated kinase, suppressing tumor growth.
- Trastuzumab (Herceptin): While not a kinase inhibitor, this monoclonal antibody targets the HER2/neu receptor, which is overexpressed in some breast cancers. By binding to HER2, trastuzumab inhibits downstream signaling pathways, including those involving PTMs, leading to reduced tumor growth and proliferation.
- Palbociclib: This drug inhibits CDK4 and CDK6, kinases involved in cell cycle regulation. It’s used in hormone receptor-positive, HER2-negative breast cancer to halt cell cycle progression and thus inhibit cancer cell proliferation.
In summary, while challenges exist, the targeting of PTMs and their regulatory enzymes has opened new avenues in drug development, especially in oncology. Continued research into PTMs, their regulation, and their involvement in disease will likely yield even more therapeutic targets in the future.
Future Perspectives
Advancements in PTM Detection and Analysis:
1. Improved Mass Spectrometry Techniques: As mass spectrometry technology continues to evolve, it’s expected that there will be even higher resolution, sensitivity, and speed in detecting and analyzing PTMs. This will allow for better mapping of complex modifications and their dynamics.
2. Integration with Systems Biology: By integrating PTM data with systems biology approaches, researchers can build more comprehensive models of cellular signaling networks. This can help predict how changes in one PTM might ripple through and affect an entire system.
3. Enhanced Imaging Techniques: Advanced microscopy, such as super-resolution and live-cell imaging, will allow real-time visualization of PTMs in living cells, facilitating understanding of their dynamics and interactions in a native environment.
Novel PTMs and their Implications:
1. Discovery of New Modifications: Though many PTMs are well-established, there’s potential to discover novel modifications as detection methods become more sensitive. These new PTMs could provide insights into previously unexplained cellular phenomena.
2. Expanding the “Code”: Just as the concept of the “histone code” expanded our understanding of how combinations of histone modifications influence gene expression, it’s possible that other proteins operate under similar “codes” defined by their PTM patterns.
Therapeutic Potential of Targeting Novel PTMs:
1. Expanding the Drug Target Landscape: As novel PTMs are discovered and understood, they might become targets for therapeutic intervention, broadening the scope of druggable targets especially in challenging diseases.
2. Precision Medicine: As we get a more refined understanding of PTMs, it may be possible to develop drugs tailored to specific PTM patterns seen in individual patients. This can lead to more personalized and effective treatments.
3. Modulating PTMs for Disease Prevention: By understanding the role of PTMs in early disease onset or progression, there might be opportunities to develop treatments that modulate PTMs as preventive measures.
4. Synthetic Biology and PTMs: As the field of synthetic biology grows, understanding and harnessing PTMs will be crucial. Designing proteins with specific PTMs can aid in building biological systems for therapeutic or industrial purposes.
In conclusion, the future of PTM research is promising. With advancements in detection and analysis methods, the discovery of novel modifications, and the increasing realization of their therapeutic potential, PTMs will remain at the forefront of molecular and cellular biology research and its applications in medicine.
Conclusion
Post-translational modifications (PTMs) stand as a testament to the intricate and dynamic nature of cellular processes. They underscore the idea that the life of a protein extends far beyond its synthesis. From a single gene, the proteome’s complexity is immensely expanded not just by the variety of proteins synthesized but also by the myriad ways they can be modified after synthesis.
From simple phosphorylation events that can toggle a protein’s activity to intricate glycosylation patterns influencing protein stability and signaling, PTMs contribute to almost every cellular process. They play pivotal roles in cell signaling, protein localization, and interaction dynamics. When appropriately regulated, PTMs maintain cellular homeostasis, but when dysregulated, they can drive disease processes, including cancer, neurodegenerative disorders, and metabolic diseases.
The diversity and complexity of PTMs offer both challenges and opportunities. While their multifaceted nature can make them difficult to study and characterize, their central role in cellular processes makes them highly attractive as therapeutic targets.
As we’ve journeyed through the world of PTMs, from their discovery and analysis to their implications in health and disease, one thing is clear: our current understanding, as vast as it might seem, is just the tip of the iceberg. With advancements in technology and analytical techniques, novel PTMs await discovery, and with them, fresh insights into cellular processes and new therapeutic possibilities.
For budding scientists, established researchers, and curious minds, the realm of PTMs offers a rich tapestry of questions waiting to be unraveled. The encouragement is clear: Dive deep, explore with fervor, and contribute to the ever-expanding ocean of knowledge. In understanding and harnessing the power of PTMs, we move closer to unlocking mysteries of biology and crafting innovative solutions for the challenges of medicine.