Introduction to Glycomics
March 20, 2024Table of Contents
Course Overview:
This course will provide an introduction to the field of glycomics, which focuses on the study of the structure, function, and biology of carbohydrates (glycans) in biological systems. Students will learn about the importance of glycans in various biological processes, as well as the techniques and tools used in glycomics research.
Prerequisites:
Basic knowledge of biochemistry and molecular biology.
Introduction to Glycome and Glycomics
Definition and scope of glycomics
Glycomics is the study of the structure, function, and biology of carbohydrates (glycans) in biological systems. It involves the comprehensive analysis of glycomes, which are the entire complement of glycans in an organism, cell, tissue, or biological sample. Glycans are complex molecules composed of sugars (monosaccharides) that are linked together in specific patterns and can be attached to proteins (glycoproteins), lipids (glycolipids), or other molecules.
The scope of glycomics includes:
- Glycan Structure Determination: Analyzing the composition, sequence, and linkage patterns of glycans in biological samples.
- Glycan Profiling: Identifying and quantifying the diverse range of glycans present in biological samples.
- Glycoprotein and Glycolipid Analysis: Studying the glycosylation patterns of proteins and lipids, including the identification of glycosylation sites.
- Glycan Function and Interaction Studies: Investigating the role of glycans in biological processes such as cell signaling, immune response, and pathogen-host interactions.
- Glycan Biomarker Discovery: Identifying glycans that can serve as biomarkers for disease diagnosis, prognosis, and monitoring.
- Glycoengineering: Modifying glycans for various applications, such as developing therapeutic glycoproteins or vaccines.
Glycomics is a multidisciplinary field that combines techniques from biochemistry, molecular biology, mass spectrometry, chromatography, and bioinformatics. It plays a crucial role in understanding the complex roles of glycans in health and disease, as well as in the development of glycan-based therapeutics and diagnostics.
What is the glycome and its importance
The glycome refers to the complete set of glycans (carbohydrates) in a cell, tissue, organism, or biological sample. It includes all the glycan structures, their different forms, and the variations in their compositions and linkages. The glycome is highly diverse and complex, with a wide range of structures and functions.
The importance of the glycome lies in several key areas:
- Cellular Recognition and Signaling: Glycans play crucial roles in cell-cell recognition, cell adhesion, and cell signaling. They are involved in processes such as immune response, embryonic development, and tissue regeneration.
- Protein Function and Stability: Glycans can modulate the structure, stability, and function of glycoproteins. They are important for protein folding, trafficking, and targeting.
- Disease Biomarkers: Changes in the glycome have been associated with various diseases, including cancer, diabetes, and autoimmune disorders. Glycans can serve as biomarkers for disease diagnosis, prognosis, and monitoring.
- Microbial Pathogenesis: Many pathogens, including bacteria and viruses, use glycans to interact with host cells and evade the immune system. Understanding the glycome of pathogens is important for developing therapies and vaccines.
- Drug Development: Glycans are increasingly being targeted for drug development. Therapeutic glycoproteins, such as antibodies and enzymes, are glycosylated to improve their stability, efficacy, and half-life in the body.
- Biotechnology and Materials Science: Glycans have applications in biotechnology and materials science. They are used in the production of vaccines, biomaterials, and glycoconjugate vaccines.
Overall, the glycome is a complex and dynamic entity that plays diverse and critical roles in biology, health, and disease. Studying the glycome is essential for understanding these roles and developing new therapies and diagnostics.

Historical perspective and milestones in glycomics research
Glycomics, the study of glycans in biological systems, has a rich history that has evolved over several decades. Here are some key milestones and developments in the field of glycomics research:
- Early Glycobiology: The study of glycans began in the early 20th century with the discovery of blood group antigens by Karl Landsteiner and the identification of glycoproteins and glycolipids in the 1930s and 1940s.
- 1970s-1980s: During this period, researchers began to investigate the structure and function of glycans in more detail. Techniques such as lectin affinity chromatography and enzymatic deglycosylation were developed to study glycan-protein interactions and glycan structures.
- 1990s: The field of glycobiology expanded rapidly in the 1990s with the development of new technologies, such as mass spectrometry and glycan microarrays, which allowed for the high-throughput analysis of glycans.
- 2000s: The term “glycomics” was coined in the early 2000s to describe the systematic study of the glycome. This period saw significant advances in glycomics research, including the development of glycan databases, such as GlycoBase and GlyTouCan, and the establishment of the Consortium for Functional Glycomics (CFG) to promote glycomics research.
- Recent Advances: In recent years, glycomics research has continued to advance, with a focus on understanding the role of glycans in health and disease. Advances in technologies such as glycoproteomics, glycoengineering, and computational glycomics have further expanded our knowledge of the glycome.
- Clinical Relevance: Glycomics research has important clinical implications, particularly in the fields of cancer, infectious diseases, and immune disorders. Glycan biomarkers are being investigated for their potential use in disease diagnosis, prognosis, and treatment.
Overall, glycomics has undergone significant growth and development since its inception, leading to a greater understanding of the role of glycans in biology and opening up new avenues for research and therapeutic development.
Structure and Diversity of Glycans
Basic structure of glycans: monosaccharides, oligosaccharides, and polysaccharides
Glycans, also known as carbohydrates or saccharides, are molecules composed of carbon, hydrogen, and oxygen atoms. They can be classified into three main categories based on their size and complexity:
- Monosaccharides: Monosaccharides are the simplest form of carbohydrates and cannot be hydrolyzed into smaller units. They are the building blocks of more complex carbohydrates. Common monosaccharides include glucose, fructose, and galactose.
- Oligosaccharides: Oligosaccharides consist of a small number (typically 2 to 10) of monosaccharide units joined together by glycosidic bonds. They are often found as components of glycoproteins, glycolipids, and other complex carbohydrates. Examples include lactose (composed of glucose and galactose) and sucrose (composed of glucose and fructose).
- Polysaccharides: Polysaccharides are complex carbohydrates composed of many monosaccharide units joined together. They can be linear or branched, and their functions vary widely. Examples of polysaccharides include starch (a storage form of glucose in plants), glycogen (a storage form of glucose in animals), and cellulose (a structural component of plant cell walls).
The structure of glycans can vary greatly depending on the types of monosaccharides present, the linkages between them, and the branching patterns. This structural diversity is essential for the diverse functions that glycans perform in living organisms, including cell-cell recognition, signaling, and structural support.
Glycan biosynthesis and processing
Glycan biosynthesis and processing are complex processes involving the synthesis, modification, and degradation of glycans in cells. These processes are tightly regulated and involve a series of enzymes and pathways. Here’s an overview of glycan biosynthesis and processing:
- Synthesis of Glycan Precursors:
- Glycan biosynthesis begins with the synthesis of nucleotide sugar precursors, such as UDP-glucose, UDP-galactose, and GDP-mannose, which serve as the building blocks for glycans.
- Assembly of Glycans:
- Glycans are assembled stepwise on glycan precursors, typically on lipid or protein carriers in the endoplasmic reticulum (ER) and Golgi apparatus.
- Enzymes called glycosyltransferases catalyze the addition of monosaccharides to the growing glycan chain, forming specific glycosidic linkages.
- Glycan Modification:
- After assembly, glycans can undergo various modifications, such as trimming, branching, and addition of functional groups.
- These modifications are carried out by glycosidases, glycosyltransferases, and other enzymes, resulting in the diversity of glycan structures.
- Glycan Processing:
- Processed glycans are transported to their final destination within the cell or to the cell surface.
- Glycans on glycoproteins and glycolipids can undergo further processing in the Golgi apparatus, including trimming, branching, and addition of terminal sugars.
- Degradation of Glycans:
- Glycans can be degraded by glycosidases, enzymes that cleave glycosidic bonds between sugar units.
- Degradation products are often recycled or used as energy sources by cells.
- Regulation of Glycan Biosynthesis:
- Glycan biosynthesis and processing are tightly regulated at the transcriptional, translational, and post-translational levels.
- Changes in glycan biosynthesis can have profound effects on cell function, development, and disease.
Overall, glycan biosynthesis and processing are highly regulated processes essential for the proper functioning of cells and organisms. Dysregulation of these processes can lead to various diseases, including cancer, diabetes, and neurodegenerative disorders.
Structural diversity of glycans and its biological significance
The structural diversity of glycans is immense, surpassing that of nucleic acids and proteins. This diversity arises from the various monosaccharides, linkages, branching patterns, and modifications that can occur in glycans. The biological significance of this diversity is profound and impacts a wide range of biological processes. Here are some key aspects of the structural diversity of glycans and their biological significance:
- Cellular Recognition and Adhesion: Glycans are involved in cell-cell recognition and adhesion processes. The diverse structures of glycans allow for specific interactions between cells, which are crucial for immune responses, tissue development, and host-pathogen interactions.
- Protein Function and Stability: Glycans are often attached to proteins (glycoproteins) and can affect their structure, stability, and function. Glycan structures can influence protein folding, localization, and interaction with other molecules.
- Cell Signaling: Glycans can act as signaling molecules by binding to specific receptors on cell surfaces. This can trigger intracellular signaling cascades that regulate processes such as cell growth, differentiation, and apoptosis.
- Pathogen Recognition and Immune Response: Many pathogens express glycans on their surfaces that mimic host glycans. This molecular mimicry can help pathogens evade the immune system. Conversely, host glycans can also serve as antigens that trigger immune responses.
- Developmental Processes: Glycans play critical roles in embryonic development, organ formation, and tissue regeneration. They are involved in cell migration, adhesion, and differentiation processes that are essential for development.
- Disease Biomarkers: Changes in glycan structures are associated with various diseases, including cancer, diabetes, and inflammatory disorders. Glycan structures can serve as biomarkers for disease diagnosis, prognosis, and monitoring.
- Drug Targeting and Delivery: The structural diversity of glycans can be exploited for drug targeting and delivery. Glycan-binding proteins, such as lectins, can be used to target drugs to specific cells or tissues.
Overall, the structural diversity of glycans is essential for a wide range of biological processes and has significant implications for health and disease. Understanding the complexity of glycan structures and their functions is crucial for advancing our knowledge of biology and developing new therapeutic strategies.
Analytical Techniques in Glycomics
Overview of analytical techniques used in glycomics, including mass spectrometry, chromatography, and glycan microarrays
Analytical techniques used in glycomics are diverse and include a range of methods for analyzing the structure, composition, and function of glycans. Some of the key techniques used in glycomics research include:
- Mass Spectrometry (MS):
- MS is a powerful technique for analyzing the mass and structure of glycans.
- MALDI-TOF MS and ESI-MS are commonly used for glycan analysis, providing information on glycan composition, sequence, and branching patterns.
- Liquid Chromatography (LC):
- LC is often coupled with MS (LC-MS) for glycan analysis, allowing for separation of complex glycan mixtures prior to mass spectrometric analysis.
- Size-exclusion chromatography (SEC), ion-exchange chromatography (IEC), and reverse-phase chromatography (RPC) are commonly used LC techniques in glycomics.
- Glycan Microarrays:
- Glycan microarrays are high-throughput platforms used to study the binding interactions between glycans and other molecules, such as proteins, antibodies, and lectins.
- They are valuable for identifying glycan-binding proteins and characterizing glycan-protein interactions.
- Capillary Electrophoresis (CE):
- CE is a technique for separating charged molecules, including glycans, based on their size and charge.
- CE can be coupled with MS (CE-MS) for glycan analysis, providing high-resolution separation and mass analysis of glycans.
- Nuclear Magnetic Resonance (NMR) Spectroscopy:
- NMR spectroscopy can provide detailed structural information about glycans, including the linkage positions and anomeric configurations of monosaccharides.
- NMR is particularly useful for studying the conformation and dynamics of glycans in solution.
- High-Performance Anion-Exchange Chromatography (HPAEC):
- HPAEC is a technique for separating and analyzing carbohydrates, including glycans, based on their charge properties.
- It is often used for glycan profiling and quantification.
- Glycan Sequencing:
- Glycan sequencing involves determining the precise sequence of monosaccharide residues and glycosidic linkages in a glycan.
- It can be achieved using a combination of analytical techniques, including MS, NMR, and enzymatic digestion.
These analytical techniques are essential for studying the structural diversity, composition, and function of glycans in biological systems, and they play a crucial role in advancing our understanding of glycan biology and its implications in health and disease.
Sample preparation techniques for glycomics analysis
Sample preparation is a critical step in glycomics analysis, as it can impact the quality and reliability of the results. Here are some key sample preparation techniques commonly used in glycomics:
- Glycan Release:
- Enzymatic release: Glycans are enzymatically released from glycoproteins or glycolipids using enzymes such as PNGase F (for N-glycans) or specific glycosidases (for O-glycans).
- Chemical release: Glycans can also be released chemically using reagents such as hydrazine or trifluoromethanesulfonic acid (TFMS).
- Purification:
- Glycan purification is often necessary to remove proteins, lipids, and other contaminants.
- Techniques such as solid-phase extraction, size exclusion chromatography, and hydrophilic interaction chromatography (HILIC) are commonly used for glycan purification.
- Derivatization:
- Some glycans require derivatization for improved detection or chromatographic separation.
- Derivatization techniques include fluorescent labeling, permethylation, and reductive amination.
- Fractionation:
- Fractionation techniques such as high-performance liquid chromatography (HPLC) or capillary electrophoresis (CE) can be used to separate glycans based on size, charge, or other properties.
- Size exclusion chromatography (SEC) is often used for size-based fractionation of glycans.
- Desalting and Concentration:
- Desalting and concentration steps are often performed to remove salts and concentrate the glycan sample.
- Techniques such as solid-phase extraction or ultrafiltration can be used for desalting and concentration.
- Quality Control:
- Quality control steps, such as checking for glycan purity and quantifying the amount of released glycans, are important to ensure the reliability of glycomics data.
- Storage:
- Glycan samples should be stored properly to maintain their stability and integrity.
- Samples are often stored at -20°C or below to prevent degradation.
Proper sample preparation is essential for glycomics analysis, as it can affect the sensitivity, accuracy, and reproducibility of the results. Careful consideration of sample preparation techniques is necessary to ensure the success of glycomics experiments.

Hands-on demonstration of glycomics analytical techniques
Conducting a hands-on demonstration of glycomics analytical techniques can be a valuable educational experience for students. Here’s a suggested outline for such a demonstration:
Objective: To demonstrate key techniques used in glycomics analysis, including glycan release, purification, and analysis.
Materials Needed:
- Glycoprotein or glycolipid sample
- Enzymes (e.g., PNGase F)
- Chemical reagents for glycan release (e.g., hydrazine, TFMS)
- Purification columns (e.g., solid-phase extraction, HILIC)
- Derivatization reagents (e.g., fluorescent labels)
- Fractionation equipment (e.g., HPLC, CE)
- Desalting and concentration equipment (e.g., solid-phase extraction, ultrafiltration)
- Analytical instruments (e.g., mass spectrometer, chromatography system)
Procedure:
- Glycan Release:
- Demonstrate enzymatic release of glycans from a glycoprotein using PNGase F.
- Alternatively, demonstrate chemical release of glycans using hydrazine or TFMS.
- Purification:
- Show purification of released glycans using solid-phase extraction or HILIC columns.
- Explain the principles behind each purification technique and the rationale for choosing a specific method.
- Derivatization:
- If applicable, demonstrate derivatization of glycans with fluorescent labels or other reagents.
- Discuss the importance of derivatization for enhancing detection sensitivity or improving chromatographic separation.
- Fractionation:
- Show fractionation of glycans based on size, charge, or other properties using HPLC or CE.
- Discuss the significance of fractionation in glycomics analysis and how it can help resolve complex glycan mixtures.
- Desalting and Concentration:
- Demonstrate desalting and concentration of purified glycans using solid-phase extraction or ultrafiltration.
- Emphasize the importance of desalting for removing salts that could interfere with downstream analysis.
- Analysis:
- If possible, demonstrate the analysis of glycans using a mass spectrometer or chromatography system.
- Show how to interpret glycan spectra and chromatograms to identify and quantify glycans.
Discussion Points:
- The importance of each step in glycomics analysis and how they contribute to the overall workflow.
- How different techniques complement each other to provide a comprehensive analysis of glycans.
- Challenges and considerations in glycomics analysis, such as sample preparation variability and data interpretation.
Note: Ensure that all demonstrations comply with laboratory safety guidelines and regulations. Provide guidance and support to students as they perform the hands-on activities.
Mass Spectrometry-Based Glycomics Analysis Workflow
Introduction to mass spectrometry-based glycomics analysis
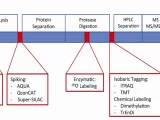
Mass spectrometry (MS) is a powerful analytical technique used in glycomics to analyze the structure, composition, and abundance of glycans. MS-based glycomics analysis involves several steps, including glycan release, purification, derivatization (if necessary), and MS analysis. Here’s an overview of mass spectrometry-based glycomics analysis:
- Glycan Release:
- Glycans are released from glycoproteins or glycolipids using enzymatic or chemical methods. PNGase F is commonly used for N-glycan release, while specific glycosidases can be used for O-glycan release.
- Purification:
- Purification of released glycans is often necessary to remove proteins, lipids, and other contaminants. Techniques such as solid-phase extraction or HILIC are commonly used for purification.
- Derivatization:
- Derivatization of glycans with fluorescent labels or other tags can enhance their detection sensitivity and improve chromatographic separation. This step is optional but can be beneficial for certain types of analyses.
- Mass Spectrometry Analysis:
- Purified glycans are analyzed using mass spectrometry to determine their mass-to-charge ratio (m/z) and provide structural information.
- Different MS techniques can be used, including matrix-assisted laser desorption/ionization (MALDI) MS, electrospray ionization (ESI) MS, and tandem MS (MS/MS).
- Interpretation of MS Data:
- MS data are analyzed to identify glycan structures based on their mass and fragmentation patterns. Software tools are used to match experimental data with glycan databases and predict glycan structures.
- Quantitative Analysis:
- MS can also be used for quantitative analysis of glycans by comparing the relative abundance of different glycan species in samples. Isotopic labeling or label-free quantification methods can be used for this purpose.
- Data Validation:
- Validation of glycan structures and quantitative data is essential to ensure the accuracy and reliability of the results. This can be done using standard reference materials and quality control samples.
Applications:
- Mass spectrometry-based glycomics analysis is used in various fields, including biomarker discovery, disease diagnosis, and understanding glycan function in biological processes.
- It provides valuable information on glycan structures, dynamics, and interactions, contributing to our understanding of glycan biology.
Overall, mass spectrometry is a versatile and powerful tool for glycomics analysis, offering high sensitivity, resolution, and the ability to analyze complex glycan mixtures.
Workflow for glycomics analysis using mass spectrometry
A typical workflow for glycomics analysis using mass spectrometry (MS) involves several key steps, including sample preparation, glycan release, purification, derivatization (if necessary), MS analysis, and data interpretation. Here’s an overview of the workflow:
- Sample Preparation:
- Start with a glycoprotein or glycolipid sample of interest.
- Extract the sample using appropriate methods and prepare it for glycan release.
- Glycan Release:
- Release glycans from glycoproteins or glycolipids using enzymatic or chemical methods.
- Common enzymes used for N-glycan release include PNGase F, while specific glycosidases are used for O-glycan release.
- Purification:
- Purify released glycans to remove proteins, lipids, and other contaminants.
- Techniques such as solid-phase extraction or hydrophilic interaction liquid chromatography (HILIC) are commonly used.
- Derivatization (Optional):
- Derivatize glycans with fluorescent labels or other tags to enhance detection sensitivity or improve chromatographic separation.
- This step is optional but can be beneficial for certain types of analyses.
- Mass Spectrometry Analysis:
- Analyze purified glycans using mass spectrometry to determine their mass-to-charge ratio (m/z) and provide structural information.
- Different MS techniques, such as matrix-assisted laser desorption/ionization (MALDI) MS or electrospray ionization (ESI) MS, can be used.
- Data Acquisition:
- Acquire MS data for the glycan samples, including MS1 spectra for mass determination and MS/MS spectra for structural elucidation.
- Data Interpretation:
- Interpret MS data to identify glycan structures based on their mass and fragmentation patterns.
- Use software tools to match experimental data with glycan databases and predict glycan structures.
- Quantitative Analysis:
- Perform quantitative analysis of glycans to compare the relative abundance of different glycan species in samples.
- Use isotopic labeling or label-free quantification methods for quantitative analysis.
- Data Validation:
- Validate glycan structures and quantitative data to ensure the accuracy and reliability of the results.
- Use standard reference materials and quality control samples for validation.
- Reporting and Publication:
- Compile the results of the glycomics analysis and prepare them for publication or presentation.
- Include details of the experimental methods, data analysis, and interpretation in the report or publication.
Overall, the workflow for glycomics analysis using mass spectrometry is a systematic process that involves multiple steps to extract, analyze, and interpret glycan data, providing valuable insights into glycan biology and function.
Data acquisition and interpretation in mass spectrometry-based glycomics analysis
In mass spectrometry-based glycomics analysis, data acquisition and interpretation are crucial steps that require careful planning and execution. Here’s an overview of the workflow for glycomics analysis using mass spectrometry, including data acquisition and interpretation:
- Sample Preparation:
- Release glycans from glycoproteins or glycolipids using enzymatic or chemical methods.
- Purify released glycans to remove proteins, lipids, and other contaminants.
- Derivatization (Optional):
- Derivatize glycans with fluorescent labels or other tags to enhance detection sensitivity.
- Mass Spectrometry Analysis:
- Analyze purified glycans using mass spectrometry.
- Use techniques such as MALDI-MS or ESI-MS to ionize glycans and generate mass spectra.
- Data Acquisition:
- Acquire mass spectra of glycans, recording their mass-to-charge ratios (m/z) and relative abundances.
- Perform tandem mass spectrometry (MS/MS) to fragment glycans and obtain structural information.
- Data Interpretation:
- Interpret mass spectra to identify glycan structures based on their m/z values and fragmentation patterns.
- Use software tools to match experimental data with glycan databases and predict glycan structures.
- Quantitative Analysis:
- Quantify glycans based on their peak intensities in mass spectra.
- Use isotopic labeling or label-free quantification methods for quantitative analysis.
- Data Validation:
- Validate glycan structures and quantitative data using standard reference materials and quality control samples.
- Verify glycan assignments using statistical analysis and validation methods.
- Biological Interpretation:
- Interpret glycomics data in the context of biological processes and pathways.
- Identify glycan biomarkers or structural features associated with disease or physiological states.
- Reporting and Documentation:
- Document experimental procedures, instrument parameters, and data analysis methods.
- Prepare reports summarizing glycomics findings and conclusions.
- Data Sharing:
- Share glycomics data with the scientific community through public databases or publications.
In summary, data acquisition and interpretation are critical steps in mass spectrometry-based glycomics analysis, providing valuable insights into glycan structures, functions, and biological roles. Proper execution of these steps ensures the reliability and reproducibility of glycomics data.
Data Analysis in Glycomics
Data processing and analysis in glycomics
Data processing and analysis in glycomics involve several steps to extract meaningful information from mass spectrometry (MS) or other analytical data. Here’s an overview of the typical workflow:
- Peak Picking:
- Identify peaks corresponding to glycan ions in the mass spectra.
- Determine peak intensities, which represent the abundance of each glycan species.
- Baseline Correction:
- Correct for baseline noise to improve the accuracy of peak identification and quantification.
- Normalization:
- Normalize peak intensities to correct for variations in sample concentration or instrument response.
- Peak Alignment:
- Align peaks across different samples or datasets to ensure accurate comparison and analysis.
- Glycan Identification:
- Match glycan masses and fragmentation patterns with databases or reference spectra to identify glycan structures.
- Use software tools to predict glycan structures based on experimental data.
- Quantification:
- Quantify glycan abundances based on peak intensities.
- Use internal or external standards for absolute quantification, or perform relative quantification by comparing peak intensities between samples.
- Statistical Analysis:
- Use statistical methods to identify significant differences in glycan abundances between groups or conditions.
- Perform clustering or multivariate analysis to identify patterns or relationships in glycan profiles.
- Annotation and Visualization:
- Annotate identified glycans with relevant information such as glycan type, linkage, and biological function.
- Visualize glycan profiles using plots, heatmaps, or other graphical representations.
- Biological Interpretation:
- Interpret glycomics data in the context of biological processes or pathways.
- Identify glycan biomarkers or structural features associated with disease or physiological states.
- Quality Control:
- Apply quality control measures to ensure the reliability and reproducibility of glycomics data.
- Include replicate analyses, standard reference materials, and other controls in data processing and analysis.
- Reporting and Documentation:
- Document data processing and analysis procedures, including software tools and parameters used.
- Prepare reports summarizing glycomics findings and conclusions.
Data processing and analysis in glycomics are critical for extracting meaningful insights from complex glycan datasets. Proper execution of these steps is essential for accurate and reliable glycomics research.

Glycomics databases and bioinformatics tools for data interpretation
In glycomics, several databases and bioinformatics tools are available to assist in data interpretation and analysis. These resources provide information on glycan structures, glycosylation sites, biosynthesis pathways, and more. Here are some commonly used databases and tools:
- GlyTouCan: A glycan structure repository that assigns a unique accession number (GlyTouCan ID) to each glycan structure, facilitating glycan annotation and data sharing.
- GlycoWorkbench: A software tool for the annotation and structural analysis of glycans. It allows users to visualize and compare glycan structures, as well as predict glycan fragmentation patterns.
- UniCarbKB: A curated database of glycan structures, glycoproteins, and glycosylation sites. It provides annotations and experimental data on glycan structures from various sources.
- GlyConnect: A database of glycoproteins and glycan structures, with a focus on mammalian glycosylation. It provides information on glycosylation sites, glycan structures, and glycoprotein functions.
- GlycomeDB: A database of glycan structures, including information on glycan composition, linkage, and source organism. It provides tools for glycan structure search and comparison.
- GlycoEpitope: A database of glycan-binding proteins (lectins) and their binding specificities. It provides information on glycan-lectin interactions and can help in glycan structure analysis.
- Glycosciences.de: A comprehensive resource for glycoscience research, including databases, tools, and educational materials. It provides access to various glycomics databases and bioinformatics tools.
- GlycoSuiteDB: An older database containing glycan structures and related information. While not actively maintained, it still provides valuable data for glycomics research.
These databases and tools are valuable resources for glycomics researchers, providing access to curated glycan structures, glycoprotein information, and bioinformatics tools for data interpretation and analysis.
Hands-on session on glycomics data analysis
Conducting a hands-on session on glycomics data analysis can be an engaging and informative experience for students. Here’s a suggested outline for such a session:
Objective: To analyze glycomics data using bioinformatics tools and databases.
Materials Needed:
- Glycomics data (e.g., mass spectra, glycan structures)
- Computer with internet access
- Bioinformatics tools and databases (e.g., GlyTouCan, UniCarbKB, GlycoWorkbench)
Procedure:
- Introduction to Glycomics Data:
- Provide an overview of glycomics data, including mass spectra and glycan structures.
- Explain the importance of data analysis in glycomics research.
- Accessing Glycomics Databases:
- Demonstrate how to access and navigate glycomics databases such as GlyTouCan and UniCarbKB.
- Show how to search for specific glycan structures or glycoproteins.
- Glycan Structure Analysis:
- Show how to analyze glycan structures using bioinformatics tools like GlycoWorkbench.
- Demonstrate how to compare glycan structures and predict fragmentation patterns.
- Glycoprotein Analysis:
- Discuss how glycoprotein data can be analyzed in the context of glycomics.
- Show how to identify glycosylation sites and analyze glycan structures attached to glycoproteins.
- Data Interpretation:
- Guide students in interpreting glycomics data and drawing conclusions from the analysis.
- Discuss how glycomics data can be used to study biological processes and diseases.
- Hands-On Practice:
- Provide students with sample glycomics data to analyze.
- Encourage them to use bioinformatics tools and databases to interpret the data.
- Discussion and Q&A:
- Facilitate a discussion on the challenges and insights gained from glycomics data analysis.
- Allow time for questions and answers to clarify any doubts.
- Conclusion:
- Summarize the key points covered in the session.
- Encourage students to explore glycomics data further on their own.
Note: Ensure that all demonstrations comply with laboratory safety guidelines and regulations. Provide guidance and support to students as they perform the hands-on activities.
Applications of Glycomics
Role of glycans in health and disease, including cancer, infectious diseases, and autoimmune disorders
Glycans play critical roles in health and disease, influencing various biological processes such as cell signaling, immune response, and protein folding. Here’s an overview of the role of glycans in different diseases:
- Cancer:
- Altered glycosylation patterns are common in cancer cells and can promote tumor growth, invasion, and metastasis.
- Aberrant glycosylation of cell surface receptors and adhesion molecules can disrupt cell-cell interactions and promote tumor progression.
- Glycan-based biomarkers, such as altered glycosylation of specific proteins, can be used for cancer diagnosis and prognosis.
- Infectious Diseases:
- Glycans on the surface of pathogens play key roles in host-pathogen interactions, including adhesion, invasion, and immune evasion.
- Pathogens often mimic host glycans to evade immune detection, allowing them to establish infection.
- Understanding the glycan interactions between pathogens and host cells can aid in the development of vaccines and therapeutics.
- Autoimmune Disorders:
- Altered glycosylation patterns on self-antigens can trigger autoimmune responses by the immune system.
- Glycans can modulate the activity of immune cells and cytokines, influencing the progression of autoimmune diseases.
- Glycan-based therapies, such as glycosylated immunoglobulins, are being investigated for the treatment of autoimmune disorders.
- Other Diseases:
- Glycans are involved in the pathogenesis of various other diseases, including neurodegenerative disorders, cardiovascular diseases, and inflammatory conditions.
- Altered glycosylation patterns on proteins in these diseases can affect their stability, function, and clearance from the body.
Overall, glycans play diverse and crucial roles in health and disease, making them important targets for research and potential therapeutic interventions. Understanding the complex interactions of glycans in disease processes can lead to new insights into disease mechanisms and the development of novel treatments.
Glycans as biomarkers for disease diagnosis and prognosis
Glycans play crucial roles in health and disease, serving as biomarkers for disease diagnosis and prognosis. Here’s an overview of how glycans function as biomarkers in various diseases:
- Cancer:
- Aberrant glycosylation is a hallmark of cancer, leading to the expression of tumor-specific glycan structures.
- Glycan alterations on proteins and lipids are associated with cancer progression, metastasis, and drug resistance.
- Glycan biomarkers such as CA19-9 (sialyl-Lewis a) and CA125 (MUC16) are used for diagnosing and monitoring cancer.
- Infectious Diseases:
- Pathogens often express unique glycan structures that can be targeted for diagnostic purposes.
- Host glycan alterations in response to infection can also serve as biomarkers.
- For example, the detection of specific glycan structures on the surface of the influenza virus can aid in diagnosis.
- Autoimmune Disorders:
- Altered glycosylation patterns on immune cells and proteins are associated with autoimmune diseases.
- Glycan biomarkers can help differentiate between different autoimmune disorders and monitor disease progression.
- For example, changes in glycosylation of IgG antibodies are observed in rheumatoid arthritis.
- Other Diseases:
- Glycans are implicated in cardiovascular diseases, neurodegenerative disorders, and metabolic syndromes.
- Glycan biomarkers are being explored for early detection and prognosis in these diseases.
Role of Glycans as Biomarkers:
- Glycans are often more stable than proteins and nucleic acids, making them attractive biomarkers for disease.
- Changes in glycosylation patterns can reflect disease-associated alterations in cellular processes and signaling pathways.
- Glycan biomarkers can be detected using various analytical techniques, including mass spectrometry, lectin microarrays, and glycan microarrays.
Challenges:
- Glycan analysis is technically challenging due to the structural complexity and heterogeneity of glycans.
- Standardization of glycan analysis methods and data interpretation is essential for reliable biomarker discovery and validation.
In conclusion, glycans serve as valuable biomarkers for disease diagnosis and prognosis in various health conditions, offering insights into disease mechanisms and potential therapeutic targets.
Future directions and challenges in glycomics research
Future directions in glycomics research are focused on addressing key challenges and advancing the field towards improved understanding and application of glycan biology. Some of the key areas of focus and challenges in glycomics research include:
- Functional Glycomics:
- Understanding the biological functions of glycans in health and disease.
- Investigating glycan-protein interactions and their roles in cellular processes.
- Glycoengineering:
- Developing methods for engineering glycan structures to modulate cellular functions.
- Creating glycan-based therapeutics and vaccines.
- Glycan Standards and Databases:
- Establishing standardized glycan databases and analytical methods for data comparison and interpretation.
- Developing glycan standards for quality control and assay validation.
- Glycan Analysis Technologies:
- Advancing analytical techniques for high-throughput glycomics analysis.
- Improving sensitivity and specificity of glycan detection methods.
- Clinical Applications:
- Identifying glycan biomarkers for disease diagnosis, prognosis, and therapeutic monitoring.
- Developing glycan-based diagnostics and therapeutics for personalized medicine.
- Systems Glycobiology:
- Integrating glycomics data with other omics data to understand complex biological systems.
- Modeling glycan-mediated signaling pathways and cellular interactions.
- Glycan Diversity and Complexity:
- Characterizing the full diversity and complexity of glycan structures in different biological contexts.
- Understanding the role of glycan heterogeneity in biological function and disease.
Challenges in glycomics research include the complexity and heterogeneity of glycan structures, the lack of standardized analytical methods, and the need for comprehensive databases and bioinformatics tools. Overcoming these challenges will require collaboration across disciplines and continued technological advancements in glycomics research.
In conclusion, future directions in glycomics research are aimed at addressing these challenges to unlock the full potential of glycans in biology and medicine. Advances in glycomics are expected to lead to new insights into disease mechanisms, novel therapeutic approaches, and improved patient care.