Proteomics and Genomics
March 30, 2024Objective of the Course :
To acquaint the student with genome organization, gene identification, expression and applications of genomics analysis. Also about proteomics, analysis and its applications.
Introduction to Genomics and Proteomics
Organization and structure of genomes
The organization and structure of genomes play a crucial role in determining an organism’s traits and functions. Genomes are the complete set of genetic material, including all the genes and non-coding sequences, present in an organism’s cells. Understanding how genomes are organized and structured is fundamental in various biological disciplines, including genetics, genomics, and bioinformatics.
Genomes can vary greatly in size and complexity across different organisms. For example, the human genome consists of approximately 3 billion base pairs of DNA, while the genome of a bacterium may contain only a few million base pairs. Despite this variation, genomes generally share a similar basic structure, consisting of genes, regulatory elements, repetitive sequences, and other functional elements.
Genes are the fundamental units of heredity and are responsible for encoding proteins, which carry out most of the biological functions in an organism. Genes are typically composed of coding regions, called exons, which are interspersed with non-coding regions, called introns. Regulatory elements, such as promoters and enhancers, control the expression of genes by influencing the rate at which they are transcribed into RNA.
In addition to genes, genomes contain repetitive sequences, which are sequences that are repeated multiple times within the genome. These repetitive sequences can be classified into two main types: tandem repeats, which are repeated adjacent to each other, and interspersed repeats, which are scattered throughout the genome.
The organization and structure of genomes are not static but can vary between individuals of the same species and even within an individual’s cells. This variation can arise from genetic mutations, which are changes in the DNA sequence, as well as from epigenetic modifications, which are changes in the chemical structure of the DNA that can affect gene expression.
Advances in sequencing technologies and bioinformatics tools have revolutionized our ability to study the organization and structure of genomes. These tools have enabled researchers to sequence entire genomes quickly and cost-effectively, allowing for the comparison of genomes between different species and the identification of genetic variations associated with diseases and other traits.
In summary, the organization and structure of genomes are essential for understanding the genetic basis of traits and diseases and have broad implications for fields ranging from evolutionary biology to personalized medicine.
Genome size
Genome size refers to the total amount of DNA contained within an organism’s haploid genome, typically measured in base pairs (bp) or in kilobase pairs (kb) for larger genomes. Genome sizes can vary widely among different species, ranging from just a few thousand base pairs in some viruses to billions of base pairs in complex organisms like humans.
The size of a genome is not necessarily correlated with the complexity of an organism. For example, the genome of the unicellular amoeba Amoeba dubia is about 670 billion base pairs, making it one of the largest known genomes, even larger than the human genome. In contrast, the genome of the tiny fruit fly Drosophila melanogaster is only about 180 million base pairs, yet it is a complex multicellular organism.
Several factors contribute to the variation in genome size among organisms. One factor is the amount of non-coding DNA present in the genome. Non-coding DNA includes regions such as introns, which are spliced out during gene expression, as well as repetitive DNA sequences with no known function. The proportion of non-coding DNA can vary significantly between species, contributing to differences in genome size.
Another factor influencing genome size is the presence of repetitive DNA sequences. These sequences can make up a significant portion of some genomes. For example, the genome of maize (corn) is about 85% repetitive sequences. The size and complexity of repetitive sequences can vary greatly between species and can impact genome size.
Genome size can also be influenced by the presence of gene families and duplications. Some organisms have undergone whole-genome duplications, where the entire genome is duplicated, leading to an increase in genome size. Gene families, where multiple copies of a gene exist, can also contribute to genome size.
Advances in sequencing technologies have enabled scientists to study genome size more accurately and efficiently. Genome size estimation is important for understanding genome evolution, genetic diversity, and the biology of organisms.
Sequence complexity
Sequence complexity refers to the diversity and repetitiveness of nucleotide sequences within a genome or a specific DNA or RNA sequence. It is a measure of how many unique sequences are present and how often they are repeated.
Sequence complexity is influenced by various factors, including the size of the genome, the presence of repetitive elements, and the organization of genes and non-coding regions. Genomes with high sequence complexity contain a wide variety of unique sequences and are often associated with more complex organisms.
Sequence complexity can be quantified using different methods, such as calculating the frequency of unique k-mers (subsequences of length k) in a sequence. Higher sequence complexity is indicative of a more diverse set of genetic information, while lower sequence complexity may indicate repetitive or conserved regions.
In genomics, understanding sequence complexity is important for genome assembly, annotation, and comparative genomics studies. It can provide insights into the evolutionary history of organisms and the functional significance of different genomic regions.
Introns and Exons
Introns and exons are two types of regions found in eukaryotic genes, which are segments of DNA that encode proteins. These regions are important for gene expression and protein synthesis.
Exons are the coding regions of a gene that are transcribed into messenger RNA (mRNA) and ultimately translated into proteins. Exons contain the genetic information necessary to produce specific amino acid sequences, which are the building blocks of proteins. The number and arrangement of exons can vary among genes and can influence the structure and function of the resulting protein.
Introns, on the other hand, are non-coding regions of a gene that are transcribed into mRNA but are removed during a process called splicing before the mRNA is translated into a protein. Introns do not contain protein-coding information and were once thought to be “junk DNA.” However, it is now known that introns play important roles in gene regulation, evolution, and the generation of protein diversity.
The process of splicing, which removes introns and joins exons together, is essential for producing functional mRNA molecules. Alternative splicing, where different combinations of exons are joined together, can generate multiple mRNA transcripts from a single gene, leading to the production of different protein isoforms with distinct functions.
In summary, exons are the coding regions of genes that contain the genetic information for protein synthesis, while introns are non-coding regions that are removed during splicing. Both exons and introns play crucial roles in gene expression and protein diversity.
Genome structure in viruses and prokaryotes
The genome structure in viruses and prokaryotes differs significantly due to their distinct biological characteristics and evolutionary histories.
- Viruses:
- Viruses have a simple genome structure compared to cellular organisms. They can have either DNA or RNA as their genetic material, but not both.
- Viral genomes can be single-stranded (ss) or double-stranded (ds) and can be linear or circular.
- Some viruses have segmented genomes, where the genetic material is divided into separate molecules. Each segment often encodes different viral proteins.
- Viruses typically have compact genomes with a limited number of genes, which encode proteins necessary for viral replication and assembly.
- Viruses often utilize the host cell’s machinery for replication and gene expression.
- Prokaryotes (Bacteria and Archaea):
- Prokaryotic genomes are typically much larger and more complex than viral genomes. They consist of double-stranded DNA organized into a circular chromosome in most cases.
- Some prokaryotes may have linear chromosomes, but this is less common.
- Prokaryotic genomes may also contain extrachromosomal DNA elements called plasmids, which can replicate independently of the chromosome.
- Prokaryotic genomes can be highly variable in size, ranging from a few hundred thousand base pairs to several million base pairs.
- Prokaryotic genomes contain genes encoding proteins, as well as regulatory sequences and non-coding regions that play important roles in gene regulation and genome organization.
In summary, viruses have relatively simple genomes that are optimized for efficient replication within host cells, while prokaryotes have larger and more complex genomes that encode a wide range of functions necessary for their independent cellular life.
Isolation of Chromosomes
The isolation of chromosomes from cells is a process used in genetics and molecular biology to study the structure, function, and organization of chromosomes. Chromosomes contain an organism’s genetic material and are composed of DNA and associated proteins.
The isolation of chromosomes typically involves several steps:
- Cell Lysis: Cells are broken open to release their contents, including the chromosomes. This can be achieved using mechanical disruption, such as grinding or homogenization, or by using chemical or enzymatic methods to break down the cell membrane and cell wall.
- Nuclear Isolation: The nuclei of the cells are isolated from the cell lysate. This can be done by differential centrifugation, where the cell lysate is centrifuged at different speeds to separate the nuclei from other cellular components.
- Chromosome Extraction: The nuclei are treated with enzymes, such as proteases and nucleases, to remove proteins and RNA, leaving behind the chromosomes. The chromosomes can then be extracted using methods such as density gradient centrifugation or filtration.
- Purification: The extracted chromosomes are further purified to remove any remaining contaminants. This can be done using additional centrifugation steps or by using chromatography techniques.
- Analysis: The isolated chromosomes can be analyzed using various techniques, such as microscopy, to study their structure and organization, or by molecular biology techniques, such as DNA sequencing or Southern blotting, to study their genetic content.
The isolation of chromosomes is a critical step in many genetic and genomic studies, as it allows researchers to study the genetic material of an organism in detail and to investigate the role of chromosomes in inheritance, development, and disease.
chromosome micro dissection
Chromosome microdissection is a technique used to isolate specific regions of a chromosome for further analysis. It allows researchers to study the genetic material within a particular chromosomal region, which can be useful for identifying genes associated with specific traits or diseases, studying chromosomal abnormalities, and understanding genome organization.
The process of chromosome microdissection involves several steps:
- Preparation of Chromosomes: Chromosomes are isolated from cells using standard methods, such as cell lysis and centrifugation, to obtain a suspension of chromosomes.
- Microdissection: A microscope equipped with micromanipulation tools is used to select and isolate the desired chromosomal region. This is typically done by using a microneedle to cut out the region of interest from the chromosome under microscopic guidance.
- Transfer of Isolated DNA: The isolated chromosomal region is transferred to a collection tube containing a buffer solution. The DNA from the isolated region can then be amplified using techniques such as polymerase chain reaction (PCR) for further analysis.
- Analysis: The isolated DNA can be analyzed using various molecular biology techniques, such as DNA sequencing, fluorescent in situ hybridization (FISH), or microarray analysis, to study its genetic content and identify any genes or genetic markers present in the region.
Chromosome microdissection is a powerful tool for studying chromosomal structure and function, and it has been used in a wide range of research areas, including cancer genetics, developmental biology, and evolutionary biology. It allows researchers to isolate and study specific chromosomal regions with high precision, providing valuable insights into the genetic basis of various biological processes.
Retrofitting
Retrofitting generally refers to the process of adding new technology or features to older systems or structures to improve their functionality, performance, or efficiency. In the context of bioinformatics or genomics, retrofitting could refer to updating or enhancing existing computational tools, algorithms, or databases to meet new requirements or to take advantage of advances in technology.
For example, in bioinformatics, retrofitting could involve updating a software tool for analyzing genetic sequences to support new file formats or to improve its speed and accuracy. In genomics, retrofitting might involve updating a database of genetic information to include new data or to improve its accessibility and usability.
Retrofitting is often necessary to keep pace with the rapid advancements in technology and to ensure that bioinformatics and genomics tools remain relevant and effective. It can also help extend the life of existing systems and structures, reducing the need for costly replacements.
Introduction to Proteomics – The Proteome
Proteomics is the large-scale study of proteins, particularly their structures and functions. It aims to understand the complete set of proteins, known as the proteome, produced by an organism or a system under specific conditions. The proteome is dynamic and can vary depending on factors such as cell type, developmental stage, and environmental conditions.
Proteins are essential molecules that perform a wide range of functions in living organisms, including catalyzing biochemical reactions, providing structural support, and regulating gene expression. Studying the proteome can provide insights into how proteins function individually and in complex networks, shedding light on biological processes and disease mechanisms.
The field of proteomics has evolved rapidly with advancements in technology, particularly in mass spectrometry and protein separation techniques. These technologies allow researchers to identify and quantify thousands of proteins in a single experiment, enabling comprehensive studies of protein expression, post-translational modifications, and protein-protein interactions.
In addition to mass spectrometry, other techniques commonly used in proteomics include protein microarrays, which allow for high-throughput analysis of protein interactions, and protein crystallography, which provides detailed structural information about proteins.
Proteomics has applications in various fields, including medicine, where it is used for biomarker discovery, drug development, and personalized medicine. In agriculture, proteomics can help improve crop yields and resistance to pests and diseases. In environmental science, proteomics can be used to study microbial communities and their interactions with the environment.
Overall, proteomics plays a crucial role in advancing our understanding of biology and has the potential to revolutionize fields such as medicine, agriculture, and environmental science.
Mining proteomes
Mining proteomes refers to the process of extracting valuable information from large-scale datasets of proteins, known as proteomes. Proteomes encompass all the proteins produced by an organism or a biological system under specific conditions.
There are several approaches and techniques used in proteome mining:
- Protein Identification: One of the primary goals of proteome mining is to identify the proteins present in a sample. This is often done using mass spectrometry, which can analyze complex mixtures of proteins and identify them based on their mass and charge.
- Protein Quantification: Proteome mining also involves quantifying the abundance of proteins in a sample. This can provide insights into the relative expression levels of different proteins and their roles in biological processes.
- Post-Translational Modification (PTM) Analysis: Proteome mining includes studying post-translational modifications (PTMs) of proteins, such as phosphorylation, glycosylation, and acetylation. PTMs can have significant effects on protein function and are important for understanding protein regulation.
- Protein-Protein Interaction (PPI) Analysis: Proteome mining involves studying the interactions between proteins, known as protein-protein interactions (PPIs). This can help elucidate the roles of proteins in biological pathways and networks.
- Functional Annotation: Proteome mining includes annotating the functions of proteins based on their sequence, structure, and known interactions. This can help assign biological roles to proteins and understand their contributions to cellular processes.
- Comparative Proteomics: Proteome mining often involves comparing proteomes across different conditions, such as healthy vs. diseased tissues or treated vs. untreated cells. This can help identify proteins that are differentially expressed or modified under specific conditions.
Overall, proteome mining is a powerful approach for understanding the complex biology of organisms and biological systems. It provides insights into protein function, regulation, and interactions, and has applications in fields such as medicine, agriculture, and biotechnology.
Bridging Genomics and Proteomics
Bridging genomics and proteomics involves integrating data and knowledge from both fields to gain a comprehensive understanding of biological systems. Genomics focuses on the study of genes and their functions, while proteomics focuses on the study of proteins and their roles in biological processes. By combining genomics and proteomics, researchers can elucidate how genes are translated into proteins and how these proteins function in cells and organisms.
There are several ways in which genomics and proteomics can be bridged:
- Gene Expression Studies: Genomics can identify genes that are transcribed under specific conditions, while proteomics can identify proteins that are synthesized as a result of gene expression. By comparing gene expression data with protein expression data, researchers can validate gene predictions and gain insights into the regulation of gene expression.
- Protein Identification and Annotation: Genomics can provide a blueprint of the proteins encoded by an organism’s genome, while proteomics can identify and annotate these proteins. Integrating genomic and proteomic data can help improve the accuracy of protein identification and annotation.
- Functional Analysis: Genomics can predict the functions of genes based on sequence similarity to known genes, while proteomics can provide direct evidence of protein function. By combining genomic and proteomic data, researchers can validate gene function predictions and discover novel functions for uncharacterized genes.
- Pathway and Network Analysis: Genomics can identify genes that are part of biological pathways and networks, while proteomics can identify proteins that interact with each other within these pathways and networks. Integrating genomic and proteomic data can help reconstruct comprehensive biological pathways and networks.
- Disease Biomarker Discovery: Genomics can identify genetic variants associated with disease, while proteomics can identify proteins that are differentially expressed or modified in disease states. By integrating genomic and proteomic data, researchers can discover novel biomarkers for disease diagnosis and prognosis.
Overall, bridging genomics and proteomics is essential for gaining a holistic understanding of biological systems and advancing our knowledge of gene function, protein regulation, and disease mechanisms.
Proteomics and the new biology
Proteomics, the large-scale study of proteins, is at the forefront of the “new biology,” a term that reflects the transformative impact of proteomics on our understanding of biological systems. The new biology emphasizes a shift from the traditional reductionist approach, which focuses on studying individual genes or proteins, to a more holistic and integrative view of biology that considers the complexity and interconnectedness of biological systems.
Proteomics is driving the new biology in several ways:
- Systems Biology: Proteomics is a key component of systems biology, which aims to understand how biological systems function as a whole. Proteomics provides insights into the interactions between proteins, their roles in cellular processes, and how these processes are regulated.
- Functional Genomics: Proteomics complements genomics by providing information on protein function and regulation. By integrating proteomic and genomic data, researchers can gain a more comprehensive understanding of how genes are translated into proteins and how these proteins function in cells.
- Personalized Medicine: Proteomics is paving the way for personalized medicine by enabling the identification of biomarkers for disease diagnosis, prognosis, and treatment. Proteomic studies of patient samples can provide valuable insights into disease mechanisms and help tailor treatment strategies to individual patients.
- Drug Discovery: Proteomics is revolutionizing drug discovery by enabling the identification of new drug targets and the development of targeted therapies. Proteomic studies can reveal the effects of drugs on protein expression and function, leading to the development of more effective and personalized treatments.
- Biotechnology: Proteomics is driving advancements in biotechnology by enabling the engineering of proteins with specific functions. Proteomic studies can reveal the structure-function relationships of proteins, leading to the design of novel proteins with desired properties.
Overall, proteomics is playing a central role in shaping the new biology, which emphasizes a holistic and integrative approach to understanding biological systems. By providing insights into the complex world of proteins, proteomics is revolutionizing our understanding of biology and opening up new avenues for advancements in medicine, biotechnology, and beyond.
Gene Identification and Expression
Genome annotation
Genome annotation is the process of identifying and labeling the features of a genome, such as genes, regulatory sequences, and other functional elements. It is a crucial step in genome analysis and interpretation, as it provides information about the genetic content and organization of an organism’s genome.
Genome annotation involves several steps:
- Gene Prediction: One of the primary goals of genome annotation is to identify protein-coding genes within the genome. This is done using computational tools that scan the genome sequence for open reading frames (ORFs) and other features characteristic of protein-coding genes.
- Functional Annotation: Once genes are identified, their functions are inferred based on sequence similarity to known genes or by predicting protein domains and motifs. Functional annotation provides information about the biological roles of genes and proteins in cellular processes.
- Non-Coding RNA Annotation: Genome annotation also includes identifying and annotating non-coding RNA genes, such as transfer RNA (tRNA), ribosomal RNA (rRNA), and microRNA (miRNA) genes. These genes play important roles in gene regulation and other cellular processes.
- Regulatory Element Annotation: Genome annotation involves identifying regulatory elements, such as promoters, enhancers, and transcription factor binding sites, that control gene expression. This information is important for understanding how genes are regulated in different cell types and under different conditions.
- Repeat Annotation: Genome annotation includes identifying and annotating repetitive DNA sequences, such as transposable elements and tandem repeats. These sequences can have important functional and evolutionary implications.
- Structural Annotation: Genome annotation involves determining the structure of genes, including the locations of exons, introns, and splice sites. This information is important for understanding how genes are transcribed and spliced into functional mRNA molecules.
- Comparative Annotation: Genome annotation often involves comparing the annotated genome to other genomes to identify conserved regions and infer gene function. Comparative annotation can reveal evolutionary relationships and help identify genes that are unique to a particular species or lineage.
Genome annotation is a complex and iterative process that relies on a combination of computational analysis, experimental data, and manual curation. It is essential for understanding the genetic basis of traits and diseases, as well as for advancing our knowledge of genome evolution and function.
Traditional routes of gene identification
Traditional routes of gene identification involve several key steps, often starting with the identification of a genetic locus associated with a particular trait or disease. Here is an overview of the traditional routes of gene identification:
- Linkage Analysis: In families with a hereditary disease or trait, researchers can use linkage analysis to map the genetic locus responsible for the phenotype. This involves genotyping DNA markers throughout the genome and identifying markers that co-segregate with the trait of interest.
- Candidate Gene Approach: Once a genetic locus has been identified, researchers can use the candidate gene approach to prioritize genes within the locus for further study. This involves selecting genes based on their known function or relevance to the phenotype and analyzing their expression or function in relation to the phenotype.
- Functional Studies: To determine if a candidate gene is responsible for the phenotype, researchers can conduct functional studies, such as gene knockout or overexpression experiments in model organisms. These studies can help elucidate the role of the gene in the phenotype and validate it as a candidate gene.
- Sequencing: Once a candidate gene has been identified and validated, researchers can sequence the gene in affected individuals to identify mutations or variations that are associated with the phenotype. This can involve sequencing coding regions (exons), regulatory regions, and introns of the gene.
- Validation Studies: Finally, researchers conduct validation studies to confirm the role of the identified gene in the phenotype. This can involve studying the gene in additional affected individuals or populations, as well as functional studies to further elucidate its role.
Overall, traditional routes of gene identification rely on a combination of genetic mapping, candidate gene analysis, functional studies, and sequencing to identify and validate genes associated with specific traits or diseases. These approaches have been widely used in the past and have led to the identification of many disease-causing genes.
Detecting open-reading Frames
Detecting open-reading frames (ORFs) is an important step in genome annotation and gene prediction. ORFs are stretches of DNA or RNA that potentially encode proteins, and they are characterized by the absence of stop codons within the reading frame. Detecting ORFs involves identifying regions of a nucleotide sequence that have the potential to encode proteins.
Several methods and tools are used to detect ORFs in a genome or a nucleotide sequence:
- Six-Frame Translation: This method involves translating the nucleotide sequence in all six reading frames (three in the forward direction and three in the reverse direction) to identify potential ORFs. ORFs are identified as stretches of amino acid residues without internal stop codons.
- Minimum ORF Length: ORFs are typically defined as having a minimum length of amino acid residues to be considered as potential protein-coding regions. The minimum length can vary depending on the organism and the context.
- Conservation: ORFs that are conserved across related species are more likely to encode functional proteins. Comparative genomics can be used to identify conserved ORFs.
- Sequence Motifs: Certain sequence motifs, such as the presence of start codons (e.g., ATG) and the absence of stop codons within the ORF, can help identify potential ORFs.
- Homology Search: ORFs can be identified by comparing the nucleotide sequence to known protein sequences using tools such as BLAST (Basic Local Alignment Search Tool) to identify regions with significant similarity to known proteins.
- Hidden Markov Models (HMMs): HMMs can be used to identify ORFs based on probabilistic models of protein-coding regions. HMM profiles are built from known protein sequences and used to search for similar ORFs in a nucleotide sequence.
- Machine Learning: Machine learning algorithms can be trained on known protein-coding sequences to predict ORFs in a nucleotide sequence based on sequence features such as codon usage, GC content, and presence of start and stop codons.
Overall, detecting ORFs is a critical step in genome annotation and gene prediction, as it provides a starting point for identifying potential protein-coding genes in a genome or nucleotide sequence.
Software programs for finding genes
There are several software programs and tools available for finding genes in genomic sequences. These tools use a variety of algorithms and approaches to predict and annotate genes based on features such as open-reading frames (ORFs), sequence conservation, and gene structure. Here are some commonly used software programs for finding genes:
- GeneMark: GeneMark is a widely used gene prediction program that uses statistical models to identify protein-coding genes in genomic sequences. It is available for bacterial, archaeal, and eukaryotic genomes.
- Glimmer: Glimmer (Gene Locator and Interpolated Markov ModelER) is a gene prediction tool that uses interpolated Markov models to identify protein-coding genes in microbial genomes.
- Augustus: Augustus is a gene prediction program that uses hidden Markov models (HMMs) to predict genes in eukaryotic genomes. It is particularly useful for annotating genes in non-model organisms.
- GeneID: GeneID is a gene prediction tool that uses a combination of homology-based and ab initio methods to predict genes in genomic sequences.
- FGENESH: FGENESH is a gene prediction program that uses a hidden Markov model (HMM) to predict genes in eukaryotic genomes. It is particularly useful for annotating genes in higher eukaryotes.
- Prokka: Prokka is a software tool for annotating bacterial, archaeal, and viral genomes. It uses a combination of existing gene prediction programs and databases to annotate genes and other genomic features.
- NCBI ORFfinder: NCBI ORFfinder is a web-based tool that can be used to find ORFs in a nucleotide sequence. It is useful for identifying potential protein-coding regions in genomic sequences.
These are just a few examples of the many software programs and tools available for finding genes in genomic sequences. The choice of tool depends on the specific requirements of the analysis, such as the type of organism being studied and the desired level of annotation detail.
Identifying the function of a new gene
Identifying the function of a new gene is a critical step in understanding its role in biological processes. There are several approaches and techniques that can be used to determine the function of a new gene:
- Sequence Analysis: Sequence analysis can provide clues about the function of a gene based on its similarity to known genes. Tools such as BLAST can be used to search for similar sequences in databases and infer the function of the new gene based on homology.
- Gene Expression Analysis: Studying the expression pattern of a gene can provide insights into its function. Techniques such as RNA sequencing (RNA-seq) and quantitative real-time PCR (qPCR) can be used to measure the expression levels of the gene in different tissues and under different conditions.
- Functional Genomics: Functional genomics approaches, such as gene knockout or RNA interference (RNAi), can be used to study the effects of disrupting the new gene on cellular processes. These experiments can help elucidate the function of the gene by revealing its role in specific pathways or phenotypes.
- Protein Interaction Studies: Studying the protein interactions of the gene product can provide insights into its function. Techniques such as yeast two-hybrid screening and co-immunoprecipitation can be used to identify proteins that interact with the gene product and infer its function based on the functions of its interaction partners.
- Structural Analysis: Studying the structure of the gene product can provide insights into its function. Techniques such as X-ray crystallography and nuclear magnetic resonance (NMR) spectroscopy can be used to determine the three-dimensional structure of the protein and infer its function based on its structural features.
- Comparative Genomics: Comparing the new gene to genes in other species can provide insights into its function. Evolutionarily conserved genes are more likely to have important functions, and studying their counterparts in other species can provide clues about the function of the new gene.
- Biochemical Assays: Biochemical assays can be used to study the biochemical activity of the gene product. For example, enzyme assays can be used to determine if the gene encodes an enzyme and characterize its substrate specificity and catalytic activity.
By using these approaches in combination, researchers can gain a better understanding of the function of a new gene and its role in biological processes.
Gene ontology
Gene Ontology (GO) is a widely used bioinformatics resource that provides a structured and controlled vocabulary to describe the molecular functions, biological processes, and cellular components of genes and gene products. The GO project aims to standardize the representation of gene and gene product attributes across species and databases, making it easier to compare and analyze biological data.
The Gene Ontology is structured as a directed acyclic graph (DAG), with terms organized in a hierarchical manner. At the top level, there are three main branches:
- Molecular Function: This branch describes the elemental activities of gene products, such as binding or catalysis. Example terms include “kinase activity” and “DNA binding.”
- Biological Process: This branch describes larger, biological goals accomplished by multiple molecular activities. Example terms include “cellular respiration” and “cell cycle.”
- Cellular Component: This branch describes where gene products are active, such as in the nucleus or mitochondrion. Example terms include “nuclear envelope” and “mitochondrial matrix.”
Each term in the Gene Ontology is assigned a unique identifier (GO ID) and is associated with a definition and a list of synonyms. Genes and gene products can be annotated with GO terms to describe their functions, processes, and locations. These annotations can be used to perform enrichment analysis to identify GO terms that are overrepresented in a set of genes, providing insights into the biological significance of the gene set.
Overall, the Gene Ontology is a valuable resource for functional annotation and analysis of genes and gene products across different species and biological contexts. It is widely used in bioinformatics and genomics research to understand the functions and relationships of genes in biological systems.
Overview of comparative genomics
Comparative genomics is the study of the similarities and differences in the genetic content and organization of genomes across different species. This field has been made possible by the availability of complete genome sequences from a wide range of organisms, from bacteria to humans. Comparative genomics allows researchers to gain insights into the evolution of genomes, the functions of genes, and the mechanisms of genetic diversity and adaptation.
Here is an overview of key aspects of comparative genomics:
- Genome Evolution: Comparative genomics provides insights into the evolutionary history of species by comparing the genomes of different organisms. By identifying conserved regions and gene families, researchers can infer common ancestry and evolutionary relationships between species.
- Gene and Genome Structure: Comparative genomics allows for the study of gene and genome structure, including gene content, arrangement, and organization. By comparing gene structures across species, researchers can identify conserved genes and regulatory elements, as well as species-specific genes and genomic features.
- Functional Genomics: Comparative genomics can help infer the functions of genes based on their conservation across species. Genes that are conserved across distantly related species are more likely to have important biological functions.
- Genome Annotation: Comparative genomics can improve the annotation of genomes by transferring functional annotations from well-studied species to closely related species. This can help identify genes and regulatory elements in newly sequenced genomes.
- Evolutionary Conservation: Comparative genomics allows researchers to identify regions of the genome that are evolutionarily conserved, indicating functional importance. These regions often contain genes that play key roles in development, immunity, and other biological processes.
- Gene Duplication and Divergence: Comparative genomics can help study the processes of gene duplication and divergence, which are important drivers of evolutionary innovation and adaptation. By comparing gene families across species, researchers can infer the timing and mechanisms of gene duplication events.
- Genome Rearrangements: Comparative genomics can reveal insights into genome rearrangements, such as inversions, translocations, and duplications, which can have important implications for genome evolution and speciation.
Overall, comparative genomics is a powerful approach for studying the structure, function, and evolution of genomes. It provides a framework for understanding the genetic basis of phenotypic diversity and adaptation in living organisms.
Protein structural genomics
Protein structural genomics is a field of study that focuses on determining the three-dimensional structures of proteins on a genome-wide scale. The goal of protein structural genomics is to provide structural information for as many proteins as possible, which can help elucidate their functions and roles in biological processes.
Protein structural genomics typically involves several key steps:
- Target Selection: Proteins are selected for structural determination based on criteria such as their biological importance, evolutionary conservation, and potential biomedical relevance. Targets are often selected from sequenced genomes or transcriptomes.
- Protein Production: Selected proteins are produced in large quantities using recombinant DNA technology. This typically involves cloning the gene encoding the protein into an expression vector and expressing the protein in a suitable host organism, such as bacteria, yeast, or mammalian cells.
- Protein Purification: The expressed proteins are purified to remove contaminants and obtain a highly pure sample for structural studies. Various chromatographic and biochemical techniques are used for protein purification.
- Protein Crystallization: Purified proteins are crystallized using methods such as vapor diffusion or microbatch crystallization. Crystallization is a critical step in protein structural genomics, as it allows for the determination of the protein’s three-dimensional structure using X-ray crystallography.
- X-ray Crystallography: Crystals of the protein are subjected to X-ray diffraction analysis, which generates a diffraction pattern that can be used to determine the protein’s atomic structure. This process requires sophisticated equipment and data analysis techniques.
- Nuclear Magnetic Resonance (NMR) Spectroscopy: In addition to X-ray crystallography, NMR spectroscopy is another technique used in protein structural genomics to determine the three-dimensional structures of proteins in solution. NMR spectroscopy can provide valuable structural information for smaller proteins or proteins that do not crystallize well.
- Structure Determination and Analysis: The experimental data obtained from X-ray crystallography or NMR spectroscopy is used to determine the three-dimensional structure of the protein. The structure is then analyzed to understand its function, interactions, and potential roles in biological processes.
Protein structural genomics has the potential to greatly expand our knowledge of protein structure and function, leading to insights into the molecular basis of disease and the development of new therapeutics. It is a collaborative effort involving researchers from diverse disciplines, including biology, chemistry, physics, and computational sciences.
Determining gene function
Determining gene function through sequence comparison and conserved protein structure is a common approach in bioinformatics and functional genomics. This approach relies on the principle that genes with similar sequences or structures are likely to have similar functions. Here’s how it works:
- Sequence Comparison: Genes with similar nucleotide or amino acid sequences are likely to encode proteins with similar functions. This similarity can be detected using sequence alignment algorithms, such as BLAST (Basic Local Alignment Search Tool), which compares a query sequence against a database of known sequences to identify similar sequences.
- Homology: Homologous genes are genes that share a common ancestry, and they often have similar functions. By comparing the sequence of a newly identified gene to known sequences in databases, researchers can infer the function of the new gene based on the functions of its homologs.
- Conserved Protein Structure: Proteins with similar structures often have similar functions, even if their sequences are not highly similar. This is because protein structure is more conserved during evolution than protein sequence. Structural bioinformatics tools can be used to compare the three-dimensional structures of proteins and infer their functions based on structural similarity.
- Functional Annotation: Once a gene has been identified as homologous to a known gene or has a similar protein structure, its function can be annotated based on the known function of its homologs or structurally similar proteins. This annotation can provide valuable insights into the function of the new gene and its potential roles in biological processes.
- Experimental Validation: While sequence and structural comparison can provide valuable clues about gene function, experimental validation is often needed to confirm these predictions. Functional assays, gene knockout studies, and other experimental approaches can be used to validate the predicted function of a gene.
Overall, determining gene function through sequence comparison and conserved protein structure is a powerful approach that has been widely used to annotate genes and understand their roles in biological processes. It relies on the principle of evolutionary conservation, which states that genes with similar sequences or structures are likely to have similar functions due to their shared evolutionary history.
Global expression profiling
Global expression profiling, also known as gene expression profiling or transcriptomics, is a powerful tool used in molecular biology to study the expression levels of all genes in a biological sample simultaneously. This technique provides insights into the dynamics of gene expression under different conditions, such as development, disease, or in response to environmental stimuli.
Global expression profiling involves the following key steps:
- Sample Collection: Biological samples, such as cells, tissues, or organisms, are collected from the experimental system of interest. Samples are often collected under different experimental conditions to study changes in gene expression.
- RNA Extraction: Total RNA is extracted from the collected samples using methods that preserve the integrity of RNA molecules. RNA extraction is critical for obtaining high-quality RNA for downstream analysis.
- cDNA Synthesis: The extracted RNA is converted into complementary DNA (cDNA) using reverse transcriptase. This step is necessary because most gene expression profiling techniques are based on the analysis of cDNA rather than RNA.
- Labeling: In some gene expression profiling techniques, such as microarrays, the cDNA is labeled with fluorescent dyes to allow for the detection of gene expression levels. Each condition or sample is typically labeled with a different dye to enable comparative analysis.
- Hybridization: The labeled cDNA is hybridized to a microarray or other high-throughput platform containing probes that correspond to specific genes. The hybridization pattern indicates the relative expression levels of each gene in the sample.
- Data Analysis: The hybridized microarray is scanned, and the data is analyzed to determine the expression levels of each gene. Bioinformatics tools are used to process the data, identify differentially expressed genes, and perform statistical analysis.
- Validation: The results of global expression profiling are often validated using independent methods, such as quantitative real-time PCR (qPCR), to confirm the expression levels of selected genes.
Global expression profiling is a valuable tool for studying complex biological processes and identifying genes that are involved in specific pathways or diseases. It has applications in various fields, including medicine, agriculture, and environmental science, and has contributed to our understanding of gene regulation and function.
Traditional approaches to expression profiling
Traditional approaches to expression profiling involve methods that allow for the measurement of the expression levels of a subset of genes in a biological sample. These approaches are often used to study the expression of specific genes or pathways under different conditions. Some of the traditional approaches to expression profiling include:
- Northern Blotting: Northern blotting is a technique used to study gene expression by detecting RNA transcripts in a sample. In this technique, RNA molecules are separated by size using gel electrophoresis, transferred to a membrane, and hybridized with a labeled probe specific to the gene of interest.
- Quantitative Real-Time PCR (qPCR): qPCR is a highly sensitive technique used to quantify the expression levels of specific genes in a sample. It involves the use of fluorescent probes that bind to the amplified DNA during PCR, allowing for real-time monitoring of the amplification process.
- Reverse Transcription PCR (RT-PCR): RT-PCR is a technique used to amplify and detect RNA molecules. It involves the conversion of RNA into complementary DNA (cDNA) using reverse transcriptase, followed by PCR amplification of the cDNA.
- In Situ Hybridization: In situ hybridization is a technique used to visualize the localization of specific RNA molecules within cells or tissues. It involves the hybridization of labeled RNA probes to complementary RNA sequences in the sample, followed by detection of the hybridized probes.
- Reporter Gene Assays: Reporter gene assays involve the use of reporter genes, such as luciferase or β-galactosidase, to study gene expression. The reporter gene is placed under the control of a promoter region of interest, and its expression is used as a measure of the activity of the promoter.
- Western Blotting: While primarily used for protein detection, Western blotting can also be used to semi-quantitatively measure protein expression levels. This technique involves separating proteins by size using gel electrophoresis, transferring them to a membrane, and detecting the target protein using specific antibodies.
These traditional approaches to expression profiling are still widely used and provide valuable information about gene expression levels in biological samples. However, they are limited in their ability to analyze large numbers of genes simultaneously, which has led to the development of high-throughput methods such as microarrays and RNA sequencing.
Analysis of RNA expression
Analysis of RNA expression involves studying the levels of RNA transcripts in a biological sample to understand gene expression patterns and regulatory mechanisms. There are several methods and techniques used for RNA expression analysis, each with its strengths and limitations. Some common methods include:
- Quantitative Real-Time PCR (qPCR): qPCR is a widely used technique for measuring the expression levels of specific RNA transcripts. It is highly sensitive and allows for the quantification of RNA levels over a wide dynamic range. qPCR can be used to validate gene expression changes identified by other methods.
- Microarrays: Microarrays are used to analyze the expression of thousands of genes simultaneously. They consist of DNA probes immobilized on a solid surface, which hybridize to complementary RNA transcripts from the sample. Microarrays can provide a comprehensive view of gene expression patterns but are limited by the need for prior knowledge of gene sequences.
- RNA Sequencing (RNA-Seq): RNA-Seq is a high-throughput sequencing technique used to quantify and profile RNA transcripts in a sample. It provides a digital measurement of gene expression levels and allows for the detection of novel transcripts and alternative splicing events. RNA-Seq is a powerful tool for studying gene expression in a wide range of biological samples.
- Northern Blotting: Northern blotting is a traditional method for analyzing RNA expression. It involves separating RNA molecules by size using gel electrophoresis, transferring them to a membrane, and hybridizing them with a labeled probe specific to the RNA of interest. Northern blotting is less sensitive than other methods but can provide information about RNA size and abundance.
- In Situ Hybridization (ISH): ISH is used to localize specific RNA transcripts within cells or tissues. It involves the hybridization of a labeled RNA probe to complementary RNA sequences in the sample, followed by visualization of the hybridized probe. ISH is useful for studying spatial patterns of gene expression.
- Ribosome Profiling (Ribo-Seq): Ribo-Seq is a technique used to study translation dynamics by sequencing the RNA fragments protected by ribosomes. It provides insights into translational regulation and can be used to identify actively translated mRNAs.
Each of these methods has its advantages and limitations, and the choice of method depends on the research question and experimental design. Combined approaches, such as integrating RNA-Seq data with other omics data, can provide a more comprehensive understanding of gene expression regulation in biological systems.
Applications of genome analysis and genomics
Genome analysis and genomics have a wide range of applications across various fields of biology, medicine, agriculture, and biotechnology. Some key applications include:
- Understanding Genetic Basis of Diseases: Genomics is used to study the genetic basis of diseases, including complex diseases such as cancer, diabetes, and cardiovascular diseases. Genome-wide association studies (GWAS) and sequencing studies help identify genetic variants associated with disease risk and progression.
- Personalized Medicine: Genomics is revolutionizing medicine by enabling personalized approaches to healthcare. Genetic information can be used to tailor treatments to individual patients based on their genetic makeup, improving treatment outcomes and reducing adverse effects.
- Pharmacogenomics: Pharmacogenomics uses genomic information to predict how individuals will respond to medications. This can help optimize drug selection and dosing, leading to more effective and safer treatments.
- Agricultural Genomics: Genomics is used in agriculture to improve crop yields, enhance disease resistance, and develop crops with desirable traits. Genomic tools are also used in livestock breeding to improve traits such as growth rate and disease resistance.
- Microbial Genomics: Genomics is used to study the genomes of bacteria, viruses, and other microorganisms. This information is used to understand microbial diversity, evolution, and pathogenesis, as well as to develop new treatments and vaccines.
- Evolutionary Genomics: Genomics is used to study the evolution of species by comparing their genomes. This helps understand the genetic basis of evolutionary processes and the relationships between different species.
- Environmental Genomics: Genomics is used to study the genetic diversity of organisms in different environments. This helps understand how organisms adapt to their environments and how they respond to environmental changes.
- Biotechnology: Genomics is used in biotechnology to develop new products and processes. For example, genomics is used in the development of genetically modified organisms (GMOs), gene editing technologies, and the production of biofuels and pharmaceuticals.
These are just a few examples of the many applications of genome analysis and genomics. As genomic technologies continue to advance, their impact on various fields is expected to grow, leading to new discoveries and applications in biology and beyond.
Analysis of Proteomes
Two-dimensional polyacrylamide gel electrophoresis
Two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) is a powerful technique used for the analysis of proteomes, which are the complete sets of proteins expressed by an organism, tissue, or cell at a given time. 2D-PAGE allows for the separation and visualization of complex mixtures of proteins based on their isoelectric point (pI) and molecular weight (MW).
Here’s how 2D-PAGE works:
- Isoelectric Focusing (IEF): In the first dimension, proteins are separated based on their pI, which is the pH at which a protein carries no net charge. Proteins are loaded onto an immobilized pH gradient (IPG) strip, which has a gradient of pH values along its length. When an electric field is applied, proteins migrate along the strip until they reach a pH equivalent to their pI, where they become electrically neutral and stop moving.
- SDS-PAGE: In the second dimension, proteins are separated based on their MW. The IPG strip from the first dimension is placed on top of a polyacrylamide gel containing SDS (sodium dodecyl sulfate), which denatures the proteins and gives them a negative charge proportional to their size. When an electric field is applied perpendicular to the direction of migration in the first dimension, proteins migrate through the gel based on their MW, with smaller proteins migrating faster than larger ones.
- Staining and Visualization: After electrophoresis, proteins in the gel are typically stained with a dye, such as Coomassie Blue or silver stain, to visualize them. The resulting 2D gel image shows spots corresponding to individual proteins, with each spot representing a different protein in the sample.
- Analysis: The 2D gel image can be analyzed using software to quantify the abundance of proteins and compare protein expression patterns between different samples. Proteins of interest can be excised from the gel for further analysis, such as protein identification by mass spectrometry.
2D-PAGE has been widely used in proteomics research for protein separation and identification. However, it has limitations, such as difficulty in resolving low-abundance proteins and proteins with extreme pI or MW values. Advances in mass spectrometry-based proteomics have complemented and in some cases replaced 2D-PAGE for comprehensive proteome analysis.
Sample Preparation
Sample preparation is a critical step in two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) and involves several key steps to ensure the successful separation and analysis of proteins. Here is an overview of the sample preparation process for 2D-PAGE:
- Sample Collection: Biological samples, such as cell lysates, tissues, or body fluids, are collected and processed to extract proteins. Samples should be handled carefully to avoid protein degradation and contamination.
- Protein Extraction: Proteins are extracted from the collected samples using lysis buffers containing detergents, chaotropic agents, and protease inhibitors to solubilize and stabilize the proteins. Various extraction methods can be used, depending on the sample type and the proteins of interest.
- Protein Quantification: The concentration of extracted proteins is determined using methods such as Bradford assay, BCA assay, or UV spectroscopy. This step ensures that the appropriate amount of protein is loaded onto the gel for optimal separation and detection.
- Protein Solubilization: Proteins are solubilized in a denaturing buffer containing detergents (e.g., SDS) and reducing agents (e.g., DTT or β-mercaptoethanol) to denature the proteins and break disulfide bonds, ensuring uniform charge distribution for subsequent separation by 2D-PAGE.
- Sample Labeling (Optional): If desired, proteins can be labeled with fluorescent dyes or radioactive isotopes for visualization and quantification purposes. Labeling can be done before or after protein solubilization.
- Sample Preparation for Isoelectric Focusing (IEF): For IEF, proteins are typically mixed with a rehydration buffer containing carrier ampholytes and loaded onto an immobilized pH gradient (IPG) strip. The IPG strip is then rehydrated, and IEF is performed to separate proteins based on their isoelectric points (pI).
- Sample Preparation for SDS-PAGE: After IEF, the IPG strip is equilibrated in a buffer containing reducing and alkylating agents to prepare the proteins for SDS-PAGE. The equilibrated strip is then placed on top of an SDS-PAGE gel for the second dimension of separation based on molecular weight (MW).
- Gel Electrophoresis: The prepared samples are subjected to electrophoresis in both dimensions. Proteins migrate through the gel based on their pI and MW, resulting in separation into discrete spots on the gel.
- Staining and Visualization: After electrophoresis, proteins in the gel are stained with a protein stain, such as Coomassie Blue or silver stain, to visualize them. The stained gel is then imaged, and the protein spots are analyzed using software for quantification and identification.
- Protein Identification: Protein spots of interest can be excised from the gel, digested with proteolytic enzymes (e.g., trypsin), and analyzed by mass spectrometry for protein identification.
Sample preparation is a critical aspect of 2D-PAGE and requires careful attention to detail to ensure the reproducibility and reliability of the results. Proper sample preparation can enhance the sensitivity and resolution of 2D-PAGE and facilitate the identification of proteins in complex biological samples.
Solubilization
Solubilization is a crucial step in sample preparation for two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) and involves the solubilization of proteins in a denaturing buffer. This step is necessary to denature the proteins, break disulfide bonds, and ensure uniform charge distribution for subsequent separation by 2D-PAGE. Here is an overview of the solubilization process:
- Denaturing Buffer: The denaturing buffer used for solubilization typically contains SDS (sodium dodecyl sulfate) and reducing agents, such as dithiothreitol (DTT) or β-mercaptoethanol. SDS denatures the proteins by binding to them and imparting a negative charge proportional to their size. The reducing agent breaks disulfide bonds, which helps to unfold the proteins and ensures uniform charge distribution.
- Sample Preparation: The biological sample, such as cell lysate or tissue extract, is mixed with the denaturing buffer to solubilize the proteins. The sample is typically incubated at an elevated temperature (e.g., 95°C) to ensure complete denaturation and solubilization of the proteins.
- Protein Quantification: After solubilization, the protein concentration in the sample is determined using methods such as Bradford assay, BCA assay, or UV spectroscopy. This step ensures that the appropriate amount of protein is loaded onto the gel for optimal separation and detection.
- Optional: Sample Labeling: If desired, proteins can be labeled with fluorescent dyes or radioactive isotopes for visualization and quantification purposes. Labeling can be done before or after solubilization, depending on the labeling method used.
- Storage: If not immediately used for 2D-PAGE, solubilized protein samples can be stored at -20°C or -80°C to prevent protein degradation.
Proper solubilization is essential for the success of 2D-PAGE and ensures that proteins are uniformly denatured and charged for separation by isoelectric focusing (IEF) and SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Care should be taken to optimize the solubilization conditions for different types of samples to achieve the best results in 2D-PAGE analysis.
Reduction
Reduction is a process used in protein sample preparation for two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) to break disulfide bonds and denature proteins. Disulfide bonds are covalent bonds between two sulfur atoms in cysteine residues that contribute to the three-dimensional structure and stability of proteins. Here is an overview of the reduction process in 2D-PAGE sample preparation:
- Purpose: The primary purpose of reduction is to break disulfide bonds in proteins, which helps unfold the proteins and ensures uniform charge distribution for subsequent separation by 2D-PAGE.
- Reducing Agents: Common reducing agents used in 2D-PAGE sample preparation include dithiothreitol (DTT) and β-mercaptoethanol. These agents donate a hydrogen atom to each sulfur atom in a disulfide bond, breaking the bond and converting the cysteine residues to thiol groups.
- Addition of Reducing Agent: The reducing agent is added to the protein sample in a denaturing buffer containing SDS (sodium dodecyl sulfate) and other components. The sample is then incubated at an elevated temperature (e.g., 95°C) to ensure complete reduction of disulfide bonds.
- Incubation: The sample is typically incubated for a short period (e.g., 5-10 minutes) to allow the reducing agent to break the disulfide bonds. The exact incubation time and temperature may vary depending on the specific protocol and the nature of the proteins being studied.
- Quenching: After reduction, the reaction is quenched by cooling the sample or adding a quenching agent to stop the reduction process and prevent reformation of disulfide bonds.
- Sample Handling: Reduced protein samples are then typically mixed with a sample buffer containing a tracking dye and loaded onto the first dimension of the 2D-PAGE gel for isoelectric focusing (IEF).
Reduction is an important step in 2D-PAGE sample preparation as it helps ensure that proteins are denatured and uniformly charged, which is essential for their separation by IEF and SDS-PAGE. Proper reduction conditions should be optimized for each sample to achieve optimal results in 2D-PAGE analysis.
Resolution
Resolution in the context of two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) refers to the ability of the technique to separate proteins based on their isoelectric point (pI) and molecular weight (MW) with high precision and clarity. A high-resolution 2D-PAGE gel allows for the clear visualization and separation of individual protein spots, which is essential for accurate quantification and identification of proteins.
Several factors influence the resolution of 2D-PAGE gels:
- Gel Composition: The composition of the polyacrylamide gel, including the percentage of acrylamide, crosslinker concentration, and buffer composition, can affect the resolution of protein separation. Higher acrylamide percentages are generally used for separating proteins of lower MW, while lower percentages are used for higher MW proteins.
- pH Gradient: The pH gradient in the first dimension of 2D-PAGE, which is typically generated using immobilized pH gradient (IPG) strips, determines the resolution of protein separation based on pI. Narrow pH gradients (e.g., pH 3-10) provide higher resolution than broader gradients (e.g., pH 3-11).
- Electrophoresis Conditions: The voltage, current, and duration of electrophoresis can impact the resolution of protein separation. Higher voltages and longer electrophoresis times can improve resolution but may also increase the risk of protein degradation.
- Sample Preparation: Proper sample preparation, including protein solubilization, reduction, and labeling (if applicable), is crucial for achieving high-resolution 2D-PAGE gels. Incomplete solubilization or reduction can lead to poor resolution and artifacts.
- Staining and Visualization: The choice of protein stain and imaging system can affect the resolution of protein spots on 2D-PAGE gels. Stains with high sensitivity and low background are preferred for clear visualization of protein spots.
- Image Analysis: Advanced image analysis software is used to analyze 2D-PAGE gels and quantify protein spots. The software should be able to accurately detect and quantify protein spots with high resolution.
Overall, achieving high resolution in 2D-PAGE requires careful optimization of gel composition, electrophoresis conditions, sample preparation, staining, and image analysis parameters. Optimization of these factors can help maximize the resolution and reproducibility of 2D-PAGE gels for protein separation and analysis.
Reproducibility of 2-DEDetecting proteins in polyacrylamide gels
Reproducibility is a key consideration in two-dimensional gel electrophoresis (2D-PAGE) as it ensures that results obtained from different experiments or samples can be compared reliably. Several factors can impact the reproducibility of 2D-PAGE, including gel preparation, sample preparation, electrophoresis conditions, staining, and image analysis. Here are some strategies to improve the reproducibility of 2D-PAGE:
- Standardized Protocols: Use standardized protocols for gel preparation, sample preparation, and electrophoresis to minimize variability between experiments. Document all steps carefully to ensure reproducibility.
- Quality Control: Perform quality control checks at each step of the experiment, including protein extraction, sample labeling (if applicable), and gel staining. Use protein standards to verify gel quality and ensure consistent results.
- Sample Handling: Handle samples carefully to avoid protein degradation or contamination. Use fresh samples whenever possible and store samples properly to maintain their integrity.
- Gel Casting: Prepare gels using accurate and precise measurements of acrylamide concentrations, crosslinker ratios, and buffer compositions. Use a degassing chamber to remove bubbles and ensure uniform gel quality.
- IPG Strip Rehydration: Rehydrate immobilized pH gradient (IPG) strips thoroughly and consistently to ensure uniform protein distribution and isoelectric focusing.
- Electrophoresis Conditions: Use consistent electrophoresis conditions, including voltage, current, and duration, to ensure reproducible separation of proteins based on molecular weight and isoelectric point.
- Staining and Imaging: Use a sensitive and reproducible staining method, such as Coomassie Blue or silver stain, and standardize imaging parameters for consistent visualization of protein spots.
- Data Analysis: Use reliable software for image analysis and quantification of protein spots. Perform statistical analysis to identify significant differences between samples and experiments.
By carefully optimizing experimental conditions and following standardized protocols, the reproducibility of 2D-PAGE can be significantly improved, leading to more reliable and consistent results in protein analysis.
Image analysis of 2-DE gels
Image analysis of two-dimensional gel electrophoresis (2-DE) gels is a crucial step in proteomics research, allowing for the quantification and comparison of protein expression levels between different samples. Here is an overview of the image analysis process for 2-DE gels:
- Image Acquisition: After 2-DE gels are run and stained, they are scanned using a gel documentation system to create digital images of the gels. The images should be of high quality with sufficient resolution for accurate analysis.
- Spot Detection: Image analysis software is used to detect protein spots on the gel images. This is typically done by identifying areas of intensity that correspond to proteins in the gel. The software may use various algorithms to detect spots, including thresholding, watershed segmentation, and spot matching.
- Spot Matching: In comparative analysis, spots from different gels are matched to determine differences in protein expression between samples. Spot matching can be challenging due to variations in gel staining, spot intensity, and gel-to-gel variability. Software tools are used to align and match spots across gels based on common landmarks or reference spots.
- Quantification: Once spots are detected and matched, the software quantifies the intensity of each spot, which corresponds to the abundance of the protein in the sample. The quantified data can be normalized to correct for variations in gel staining and protein loading.
- Statistical Analysis: Statistical tests, such as t-tests or ANOVA, are applied to the quantified data to identify proteins that are significantly differentially expressed between samples. Multiple testing corrections may also be applied to reduce the risk of false positives.
- Visualization: The results of the analysis are typically visualized using heatmaps, scatter plots, or other graphical representations to show differences in protein expression patterns between samples.
- Protein Identification: Proteins of interest can be excised from the gel, digested, and analyzed by mass spectrometry for identification. The spots on the gel can be matched to the identified proteins to confirm their identity.
Image analysis of 2-DE gels is a complex process that requires careful optimization and validation to ensure accurate and reliable results. Advances in software and technology continue to improve the speed and accuracy of image analysis, making 2-DE a valuable tool in proteomics research.
Mass spectrometry based methods for protein identification
Mass spectrometry (MS)-based methods are widely used for protein identification in proteomics research due to their high sensitivity and specificity. Here are some common MS-based methods for protein identification:
- Matrix-assisted laser desorption/ionization (MALDI) MS: MALDI-MS is a soft ionization technique that is often used in combination with time-of-flight (TOF) mass analyzers. Proteins are ionized by a laser beam and the resulting ions are accelerated into a TOF mass analyzer, which separates ions based on their mass-to-charge ratio (m/z). MALDI-MS is often used for peptide mass fingerprinting (PMF) for protein identification.
- Electrospray ionization (ESI) MS: ESI-MS is another soft ionization technique that is commonly used in tandem mass spectrometry (MS/MS) experiments. Proteins are ionized in solution and the resulting ions are introduced into a mass analyzer. ESI-MS is often used in conjunction with liquid chromatography (LC) to separate peptides prior to MS analysis, a technique known as LC-MS/MS.
- Tandem MS (MS/MS): In MS/MS, ions from a peptide are selected for fragmentation, and the resulting fragment ions are analyzed to deduce the amino acid sequence of the peptide. This information can be used to identify the protein from which the peptide originated. Common MS/MS techniques include collision-induced dissociation (CID) and higher-energy collisional dissociation (HCD).
- Database Search: The MS/MS data generated from a sample is typically searched against a protein sequence database using software tools such as Mascot, Sequest, or X!Tandem. The search results provide a list of proteins that match the observed peptide fragment ions, along with statistical measures of confidence.
- Post-translational modification (PTM) analysis: MS-based methods can also be used to identify and characterize post-translational modifications (PTMs) on proteins. By analyzing the mass shifts of peptide ions in MS/MS spectra, specific PTMs, such as phosphorylation or glycosylation, can be identified.
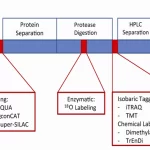
- Quantitative proteomics: MS-based methods can also be used for quantitative proteomics by comparing the abundance of proteins or peptides between different samples. This is often achieved using stable isotope labeling techniques, such as SILAC, iTRAQ, or TMT, followed by MS analysis.
MS-based methods for protein identification and quantification have revolutionized the field of proteomics, enabling researchers to study complex protein mixtures with high sensitivity and accuracy.
De novo sequencing using mass spectrometric data
De novo sequencing using mass spectrometry is a method for determining the amino acid sequence of a peptide without relying on a pre-existing protein sequence database. This approach is particularly useful for identifying novel peptides or proteins and for studying post-translational modifications. Here’s how de novo sequencing using mass spectrometry typically works:
- Peptide Ionization: The peptide of interest is ionized using an ionization technique such as electrospray ionization (ESI) or matrix-assisted laser desorption/ionization (MALDI). This creates charged peptide ions that can be analyzed by the mass spectrometer.
- Mass Analysis: The ionized peptides are then introduced into the mass spectrometer, where they are separated based on their mass-to-charge ratio (m/z). This separation allows the mass spectrometer to determine the mass of the peptide ions.
- Fragmentation: The ionized peptides are subjected to fragmentation, often using collision-induced dissociation (CID) or higher-energy collisional dissociation (HCD). This process breaks the peptide bonds within the peptide, generating a series of fragment ions.
- Fragment Ion Analysis: The mass spectrometer analyzes the masses of the fragment ions produced during fragmentation. These fragment ions contain information about the amino acid sequence of the original peptide.
- Sequence Reconstruction: By analyzing the masses of the fragment ions and their relative abundances, bioinformatics software can reconstruct the amino acid sequence of the original peptide. This process involves matching the observed fragment masses to theoretical fragment masses of all possible peptide sequences to find the best match.
- Verification: The de novo sequenced peptide sequence is typically verified by comparing it to known protein sequences or by synthesizing the peptide and confirming its sequence using techniques such as Edman degradation or tandem mass spectrometry.
De novo sequencing using mass spectrometry is a powerful tool for identifying and characterizing peptides and proteins, especially when no prior sequence information is available. However, it can be challenging, particularly for longer or highly modified peptides, and is often used in combination with database search methods for more accurate results.
Correlative mass spectrometric based identification strategies
Correlative mass spectrometric-based identification strategies combine multiple mass spectrometry (MS) techniques with other analytical methods to enhance the identification and characterization of proteins and peptides. These strategies are particularly useful for complex samples or when high confidence in protein identification is required. Here are some common correlative MS-based identification strategies:
- LC-MS/MS: Liquid chromatography-tandem mass spectrometry (LC-MS/MS) is a widely used technique for peptide and protein identification. It combines liquid chromatography to separate peptides with tandem mass spectrometry to analyze and sequence the peptides.
- MALDI-TOF/TOF: Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF/TOF) is a technique that combines MALDI ionization with TOF mass analysis. It is often used for peptide mass fingerprinting and peptide sequencing.
- LC-MS/MS with Database Search: In this strategy, peptides separated by liquid chromatography are analyzed by tandem mass spectrometry, and the resulting MS/MS spectra are searched against a protein sequence database to identify the peptides and proteins.
- LC-MS/MS with De Novo Sequencing: LC-MS/MS can also be used with de novo sequencing to identify peptides without relying on a protein sequence database. De novo sequencing is particularly useful for identifying novel peptides or for studying post-translational modifications.
- LC-MS/MS with PTM Analysis: LC-MS/MS can be used to identify and characterize post-translational modifications (PTMs) on proteins. By analyzing the mass shifts of peptide ions in MS/MS spectra, specific PTMs, such as phosphorylation or glycosylation, can be identified.
- LC-MS/MS with Quantitative Proteomics: LC-MS/MS can also be used for quantitative proteomics by comparing the abundance of proteins or peptides between different samples. This is often achieved using stable isotope labeling techniques, such as SILAC, iTRAQ, or TMT, followed by MS analysis.
- LC-MS/MS with Cross-linking: Cross-linking combined with LC-MS/MS is used to study protein-protein interactions. Cross-linkers are used to covalently link interacting proteins, and the cross-linked peptides are identified by LC-MS/MS to determine the interaction sites.
- LC-MS/MS with Ion Mobility Spectrometry (IMS): IMS can be coupled with LC-MS/MS to separate ions based on their size, shape, and charge, providing additional structural information about peptides and proteins.
2-DE gel electrophoresis coupled with mass spectrometry
Two-dimensional gel electrophoresis (2-DE) coupled with mass spectrometry (MS) is a powerful approach for protein separation, quantification, and identification in proteomics research. Here’s how the combination of these techniques works:
- 2-DE Gel Electrophoresis: In 2-DE, proteins are first separated based on their isoelectric point (pI) in the first dimension using isoelectric focusing (IEF). This separates proteins into individual spots along the gel strip based on their pI values. The gel strip is then placed on top of a polyacrylamide gel for the second dimension of separation based on molecular weight (MW) using SDS-PAGE. This separates proteins further based on their MW, resulting in a 2D gel with individual protein spots.
- Protein Staining: After electrophoresis, the proteins in the 2D gel are typically stained with a protein stain, such as Coomassie Blue or silver stain, to visualize the protein spots. Each spot on the gel corresponds to an individual protein or protein isoform.
- Spot Excision: Protein spots of interest are excised from the gel using a spot cutter or manual excision for further analysis. The gel spots are then digested into peptides using a protease, such as trypsin, to break the proteins into smaller peptides.
- Mass Spectrometry Analysis: The digested peptides are analyzed by mass spectrometry (MS) to determine their mass-to-charge ratio (m/z) and fragmentation pattern. This information is used to identify the amino acid sequence of the peptides.
- Database Search: The MS data is then searched against a protein sequence database using software tools, such as Mascot or Sequest, to identify the proteins corresponding to the peptides. The search results provide information about the identity of the proteins present in the original sample.
- Protein Identification: Proteins are identified based on the peptides identified by MS and their sequence is deduced. This information can provide insights into the identity, abundance, and post-translational modifications of proteins in the sample.
2-DE gel electrophoresis coupled with mass spectrometry is a widely used technique in proteomics research for studying complex protein mixtures, identifying proteins of interest, and understanding their roles in biological processes.
Micro array techniques
Microarray techniques are powerful tools used in genomics and proteomics research to study gene expression, protein expression, and interactions on a large scale. There are two main types of microarrays: DNA microarrays and protein microarrays.
- DNA Microarrays: DNA microarrays, also known as gene chips or DNA chips, are used to measure the expression levels of thousands of genes simultaneously. They consist of small DNA fragments, called probes, that are immobilized on a solid surface, such as a glass slide. The microarray is then hybridized with labeled cDNA or RNA samples from cells or tissues. The level of hybridization indicates the relative expression level of each gene in the sample.
- Protein Microarrays: Protein microarrays are used to study protein-protein interactions, protein expression levels, and protein function on a large scale. They consist of immobilized proteins, peptides, or antibodies on a solid surface. The microarray is then probed with fluorescently labeled proteins or antibodies to detect interactions or expression levels.
Microarray techniques offer several advantages, including:
- High-throughput: Microarrays allow for the simultaneous analysis of thousands of genes or proteins in a single experiment.
- Sensitivity: Microarrays can detect low-abundance genes or proteins that may be missed by other methods.
- Quantitative: Microarrays provide quantitative information about gene or protein expression levels.
- Discovery: Microarrays can be used to discover novel genes, proteins, or interactions that are involved in biological processes or diseases.
However, microarray techniques also have limitations, such as the need for careful experimental design, data analysis, and validation of results. Despite these limitations, microarrays remain valuable tools in genomics and proteomics research for studying complex biological systems.
Types of micorarrays
Microarrays are versatile tools used in genomics, proteomics, and other fields of biological research. They can be classified based on the type of molecules they analyze and the applications they are used for. Here are some common types of microarrays:
- DNA Microarrays: These microarrays are used to analyze the expression levels of thousands of genes simultaneously. They consist of small DNA fragments (probes) immobilized on a solid surface. DNA microarrays are used for gene expression profiling, genotyping, and comparative genomic hybridization.
- Oligonucleotide Microarrays: Oligonucleotide microarrays use short DNA sequences (oligonucleotides) as probes. They are often more specific and sensitive than traditional DNA microarrays, making them ideal for applications such as SNP detection, mutation analysis, and gene expression profiling.
- cDNA Microarrays: cDNA microarrays use complementary DNA (cDNA) as probes. They are used to measure the expression levels of genes by hybridizing cDNA samples from cells or tissues to the microarray.
- Protein Microarrays: Protein microarrays are used to study protein-protein interactions, protein expression levels, and protein function. They consist of immobilized proteins, peptides, or antibodies on a solid surface. Protein microarrays are used in drug discovery, biomarker discovery, and functional proteomics.
- Tissue Microarrays (TMAs): TMAs are used to analyze multiple tissue samples on a single slide. They consist of small tissue cores from different samples arranged in a grid pattern. TMAs are used in cancer research and pathology to study protein expression patterns in large cohorts of patients.
- Antibody Microarrays: Antibody microarrays use immobilized antibodies as capture agents to detect and quantify proteins in a sample. They are used in biomarker discovery, diagnostics, and drug development.
- Cell Microarrays: Cell microarrays are used to study cell behavior, including cell-cell interactions, cell signaling, and drug responses. They consist of immobilized cells on a solid surface and are used in drug screening and basic cell biology research.
These are just a few examples of the types of microarrays available. Each type of microarray has its advantages and limitations, and the choice of microarray depends on the specific research questions and applications.
Designing a microarray experiment
Designing a microarray experiment involves careful planning to ensure that the experiment is well-controlled, reproducible, and capable of answering the research question. Here are the key steps involved in designing a microarray experiment:
- Research Question: Clearly define the research question or hypothesis that the experiment aims to address. This will guide the selection of samples, experimental design, and data analysis.
- Sample Selection: Identify the biological samples to be analyzed in the experiment. Ensure that the samples are appropriate for the research question and that there is sufficient biological replication to provide statistical power.
- Experimental Design: Choose the appropriate type of microarray (e.g., DNA, RNA, protein) and platform based on the research question and the type of molecules to be analyzed. Determine the number of arrays needed and the labeling strategy for the samples.
- Control Samples: Include control samples in the experiment to assess the variability and reliability of the microarray data. Control samples can include reference samples, technical replicates, and spike-in controls.
- Sample Preparation: Prepare the biological samples for microarray analysis, including RNA or protein extraction, labeling, and purification. Ensure that the sample preparation methods are standardized and reproducible.
- Experimental Protocol: Develop a detailed experimental protocol that includes all steps of the microarray experiment, from sample preparation to data analysis. Include information on sample labeling, hybridization conditions, array scanning, and data normalization.
- Data Analysis Plan: Plan the data analysis approach, including methods for data preprocessing, normalization, statistical analysis, and interpretation of results. Consider using bioinformatics tools and software for data analysis.
- Validation: Validate the microarray results using independent methods, such as qPCR or Western blotting, to confirm the findings.
- Ethical Considerations: Ensure that the experiment complies with ethical guidelines and regulations, especially if human or animal samples are used.
- Reproducibility: Design the experiment to be reproducible, with sufficient technical and biological replicates to ensure the reliability of the results.
By following these steps, researchers can design a well-controlled and informative microarray experiment that provides valuable insights into gene expression, protein interactions, or other biological processes.
Microarray Technology in Treating Disease
Microarray technology has had a significant impact on the field of medicine, particularly in the diagnosis, prognosis, and treatment of diseases. Here are some ways in which microarrays are used in treating disease:
- Cancer Diagnostics: Microarrays can be used to analyze gene expression patterns in cancer cells, allowing for the identification of specific genes associated with different types of cancer. This information can be used for early detection, diagnosis, and classification of cancer, as well as for predicting patient outcomes and selecting personalized treatment options.
- Pharmacogenomics: Microarrays are used to study how genetic variations affect an individual’s response to drugs. By analyzing gene expression patterns, microarrays can help identify genetic markers that predict how a patient will respond to a particular drug, allowing for personalized treatment plans.
- Drug Discovery: Microarrays are used in drug discovery to screen large numbers of compounds for their effects on gene expression. This can help identify potential drug targets and lead compounds for further development.
- Infectious Disease Diagnostics: Microarrays can be used to detect and identify pathogens, such as bacteria and viruses, in clinical samples. They can also be used to study host-pathogen interactions and the immune response to infection.
- Biomarker Discovery: Microarrays are used to identify biomarkers, or biological indicators, of disease. By analyzing gene expression patterns in patient samples, microarrays can help identify biomarkers that can be used for early detection, diagnosis, and monitoring of disease progression.
- Personalized Medicine: Microarrays are used in personalized medicine to tailor treatment plans to individual patients based on their genetic makeup. By analyzing gene expression patterns, microarrays can help identify the most effective treatment options for each patient.
Overall, microarray technology has revolutionized the field of medicine by providing a powerful tool for studying the molecular basis of disease and developing more targeted and personalized treatment strategies.
Applications of Genomics and Proteomics Analysis
Analysis of Genomes
Analysis of genomes from different organisms, including humans, mice, Plasmodium falciparum, Saccharomyces cerevisiae, and Mycobacterium tuberculosis, involves a variety of bioinformatics tools and approaches. Here are some key aspects of genome analysis for each organism:
- Human Genome Analysis: The human genome was sequenced as part of the Human Genome Project, and subsequent studies have focused on understanding its structure, function, and variation. Analysis of the human genome involves identifying genes, regulatory elements, and genetic variations associated with diseases and traits. Tools such as BLAST, UCSC Genome Browser, and Ensembl are commonly used for human genome analysis.
- Mouse Genome Analysis: The mouse genome is often used as a model organism for studying human biology and disease. Analysis of the mouse genome involves comparative genomics to identify genes and regulatory elements that are conserved between mice and humans. Tools such as the Mouse Genome Database (MGD) and the Mouse ENCODE project are used for mouse genome analysis.
- Plasmodium falciparum Genome Analysis: Plasmodium falciparum is the parasite responsible for malaria. Analysis of the P. falciparum genome involves identifying genes involved in drug resistance, virulence, and host-parasite interactions. Tools such as PlasmoDB and GeneDB are used for P. falciparum genome analysis.
- Saccharomyces cerevisiae Genome Analysis: Saccharomyces cerevisiae, or baker’s yeast, is a model organism used in molecular biology and genetics. Analysis of the S. cerevisiae genome involves studying gene function, regulation, and evolution. Tools such as the Saccharomyces Genome Database (SGD) and YeastMine are used for S. cerevisiae genome analysis.
- Mycobacterium tuberculosis Genome Analysis: Mycobacterium tuberculosis is the bacterium that causes tuberculosis. Analysis of the M. tuberculosis genome involves studying drug resistance, pathogenesis, and host-pathogen interactions. Tools such as TubercuList and PATRIC are used for M. tuberculosis genome analysis.
In general, genome analysis involves a combination of bioinformatics tools and experimental techniques to study the structure, function, and evolution of genomes from different organisms. The goal of genome analysis is to gain a better understanding of the genetic basis of biological processes and diseases, ultimately leading to improved diagnosis, treatment, and prevention strategies.
Application of proteome analysis
Proteome analysis, the study of the entire set of proteins produced by an organism or system, has numerous applications in drug development, toxicology, and pharmaceutical research. Here are some key ways in which proteome analysis is applied in these fields:
- Drug Target Identification: Proteome analysis is used to identify potential protein targets for drug development. By studying the proteomes of diseased tissues or cells, researchers can identify proteins that are differentially expressed or modified, which may serve as targets for new drugs.
- Drug Mechanism of Action: Proteome analysis can help elucidate the mechanisms of action of drugs. By studying how drugs affect protein expression, modification, and interaction patterns, researchers can gain insights into how drugs exert their therapeutic effects.
- Biomarker Discovery: Proteome analysis is used to discover biomarkers, or biological indicators, of disease or drug response. By comparing the proteomes of healthy and diseased tissues or cells, researchers can identify proteins that are associated with specific diseases or drug responses, which can be used as biomarkers for diagnosis, prognosis, or monitoring of disease progression or drug efficacy.
- Toxicology: Proteome analysis is used in toxicology to study the effects of toxins, pollutants, and drugs on protein expression and function. By analyzing changes in the proteome in response to toxic insults, researchers can identify potential biomarkers of toxicity and gain insights into the mechanisms of toxicity.
- Pharmacokinetics and Pharmacodynamics: Proteome analysis can be used to study the absorption, distribution, metabolism, and excretion (ADME) of drugs, as well as their pharmacodynamics (PD). By analyzing how drugs affect the proteome of different tissues or cells over time, researchers can gain insights into drug metabolism, efficacy, and side effects.
- Pharmaceutical Quality Control: Proteome analysis is used in pharmaceutical quality control to ensure the consistency and purity of drug products. By analyzing the proteome of drug formulations, researchers can verify the presence of the desired proteins and detect any contaminants or impurities.
Overall, proteome analysis plays a critical role in drug development, toxicology, and pharmaceutical research by providing insights into the mechanisms of disease and drug action, identifying potential drug targets and biomarkers, and ensuring the quality and safety of drug products.
Proteomics in drug Discovery
Proteomics plays a crucial role in drug discovery by providing insights into the complex interactions between proteins and potential drug candidates. Here’s how proteomics is used in drug discovery, particularly in the context of human proteins and phage antibodies:
- Human Proteome Analysis: Proteomics is used to analyze the human proteome to identify potential drug targets and biomarkers. By studying the expression patterns, post-translational modifications, and interactions of human proteins, researchers can identify proteins that are involved in disease processes and may serve as targets for drug development.
- Target Identification and Validation: Proteomics is used to identify and validate potential drug targets. By comparing the proteomes of healthy and diseased tissues or cells, researchers can identify proteins that are specifically associated with disease and may be targeted by drugs.
- Mechanism of Action Studies: Proteomics is used to study the mechanisms of action of drugs. By analyzing how drugs affect the proteome of cells or tissues, researchers can gain insights into how drugs exert their therapeutic effects and identify potential side effects or off-target effects.
- Phage Antibodies as Tools: Phage display technology is used to generate libraries of phage antibodies (or phage display antibodies) that can bind to specific targets, including proteins involved in disease. These antibodies can be used as tools in proteomics to study protein-protein interactions, protein localization, and protein function.
- Targeted Proteomics: Targeted proteomics techniques, such as selected reaction monitoring (SRM) or multiple reaction monitoring (MRM), are used to quantify specific proteins or protein modifications. These techniques can be used to validate potential drug targets and monitor the response to drug treatment.
Overall, proteomics and phage antibodies play critical roles in drug discovery by enabling the identification of potential drug targets, elucidating the mechanisms of action of drugs, and providing tools for studying protein function and interactions.
Glycobiology and Proteomics in plant genetics and breeding
Glycobiology and proteomics play important roles in plant genetics and breeding by providing insights into the structure, function, and regulation of proteins and carbohydrates in plants. Here’s how these fields contribute to plant genetics and breeding:
- Glycobiology in Plant Cell Wall Development: Glycobiology is the study of the structure and function of carbohydrates (glycans) in biological systems. In plants, glycobiology is particularly important in understanding the structure and biosynthesis of cell wall polysaccharides, which are crucial for plant growth, development, and defense against pathogens. Understanding the role of glycans in cell wall development can lead to the development of plants with improved biomass for biofuel production or increased resistance to pathogens.
- Proteomics in Plant Breeding: Proteomics is the large-scale study of proteins, including their structure, function, and expression. In plant breeding, proteomics is used to identify proteins that are associated with desirable traits, such as yield, stress tolerance, and nutritional content. By analyzing the proteomes of different plant varieties or species, researchers can identify proteins that are linked to specific traits and use this information to guide breeding efforts.
- Marker-Assisted Breeding: Proteomics can also be used in marker-assisted breeding, where specific protein markers are used to select for desirable traits in plants. By identifying proteins that are linked to traits of interest, researchers can develop protein-based markers that can be used to screen plant populations for those traits, allowing for more efficient breeding programs.
- Stress Response and Adaptation: Proteomics is used to study how plants respond to environmental stresses, such as drought, salinity, and pathogens. By analyzing changes in the plant proteome under stress conditions, researchers can identify proteins that are involved in stress response and adaptation. This information can be used to develop plants that are more resilient to environmental stresses.
- Nutritional Improvement: Proteomics can also be used to study the nutritional content of plants. By analyzing the proteomes of different plant varieties, researchers can identify proteins that are associated with nutritional quality, such as proteins that contribute to higher levels of essential amino acids or vitamins. This information can be used to develop plants with improved nutritional value.
Overall, glycobiology and proteomics play important roles in plant genetics and breeding by providing insights into the structure, function, and regulation of proteins and carbohydrates in plants. These fields help to improve our understanding of plant biology and contribute to the development of plants with improved traits for agriculture and industry.
References
TEXT BOOKS: 1. S. B. Primrose and R.M. Twyman – Principles of Genome Analysis and Genomics, 7 th Edition, Blackwell Publishing, 2006.
2. S. Sahai – Genomics and Proteomics, Functional and Computational Aspects, Plenum Publication, 1999.
REFERENCE BOOKS:
1. Andrezej K Konopka and James C. Crabbe, Compact Hand Book – Computational Biology, Marcel Dekker, USA, 2004. 2. Pennington & Dunn – Proteomics from Protein Sequence to Function, 1 st edition, Academic Press, San Diego, 1996.