Genomics vs. Proteomics: Understanding the Key Differences
October 15, 2023Genomics vs. Proteomics
I. Introduction
A. Definition of Genomics and Proteomics
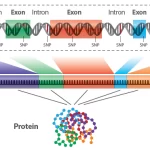
Genomics is a discipline in genetics that applies recombinant DNA, DNA sequencing methods, and bioinformatics to sequence, assemble, and analyze the structure and function of genomes (the entire set of DNA within a single cell of an organism). In essence, it revolves around the comprehensive study of genes and their roles in an organism.
Proteomics, on the other hand, is the large-scale study of proteins, particularly their structures and functions. Proteins are vital parts of living organisms, responsible for almost all of the cellular functions and structures. The term “proteomics” is derived from “PROTEin” and “genOMICS”, reflecting its relation to the field of genomics.
B. Importance in Biological Research
Both genomics and proteomics play crucial roles in biological research, offering insights that were previously unattainable.
- Understanding Diseases: Genomics has been instrumental in identifying the genes that cause diseases, allowing for early diagnosis and better treatments. For instance, the BRCA1 and BRCA2 gene mutations linked to breast cancer were identified using genomic techniques.
- Drug Development: Proteomics provides insights into protein interactions and functions, which can be pivotal in developing new and more effective drugs.
- Evolutionary Studies: Genomics can provide information on how different organisms are related and have evolved over time.
- Functional Analysis: While genomics provides the blueprint of life, proteomics gives us an understanding of the machinery. By analyzing the proteins in a cell, researchers can determine their functions and how they interact with each other.
- Personalized Medicine: Genomic information is becoming increasingly important in medical practice, paving the way for personalized medicine where treatments can be tailored to an individual’s genetic makeup.
In sum, the disciplines of genomics and proteomics are revolutionizing our understanding of biology, health, and disease, leading to breakthroughs in medicine and therapeutics.
Genomics
A. Definition and Overview
Genomics is the branch of molecular biology concerned with the study of the entire genome of organisms. The genome refers to the complete set of DNA of an organism, including all of its genes. It encompasses not only the genes themselves but also the non-coding sequences that play a role in regulating gene expression, chromosomal organization, and other vital processes.
The primary objectives of genomics include:
- DNA Sequencing: Determining the precise order of nucleotides within a DNA molecule. With advancements in technology, it has become possible to sequence entire genomes, leading to the identification of all the genes and regulatory elements in an organism.
- Genome Mapping: Creating a spatial organization of genes, often represented as a series of features on chromosomes. This helps in locating specific genes and understanding the genetic makeup of an organism.
- Functional Genomics: Understanding the function of genes and their interactions. This involves studying gene expression patterns, protein interactions, and metabolic pathways.
- Comparative Genomics: Comparing the genomes of different species to understand evolutionary relationships, identify conserved elements, and discover the genetic basis of specific traits.
- Structural Genomics: Analyzing the three-dimensional structure of proteins encoded by the genome. This helps in understanding protein function, interactions, and potential targets for drug design.
At its core, genomics focuses on DNA sequences and structure. It aims to understand how the genetic information is organized, regulated, and utilized in different cells and under various conditions. The insights gained from genomics have profound implications for medicine, agriculture, and biotechnology, paving the way for advancements in disease diagnosis, treatment, and prevention.
Methods and Technologies in Genomics
1. DNA Sequencing:
DNA sequencing is the process of determining the precise order of nucleotides (adenine, guanine, cytosine, and thymine) within a DNA molecule. Over the years, the techniques have evolved, leading to faster and more accurate sequencing methods.
- Sanger Sequencing: Developed in the 1970s by Frederick Sanger, this method involves the use of chain-terminating dideoxynucleotides. It was the primary method used in the first genome sequencing efforts, including the Human Genome Project.
- Next-Generation Sequencing (NGS): This encompasses a range of modern sequencing technologies that allow for sequencing of DNA and RNA much more quickly and cheaply than the Sanger method. Examples include Illumina sequencing, SOLiD sequencing, and 454 sequencing.
- Third-Generation Sequencing: Technologies such as PacBio’s SMRT (Single Molecule, Real-Time) sequencing and Oxford Nanopore sequencing fall into this category. They offer longer read lengths and can sequence individual DNA molecules in real-time.
2. Comparative Genomics:
Comparative genomics involves the comparison of genomes from different species to gain insights into their evolutionary relationships, identify conserved genetic elements, and understand the genetic basis of various traits.
- Whole Genome Alignment: This method involves aligning the entire genome sequences of two or more species to identify regions of similarity and difference.
- Ortholog Identification: Finding genes in different species that evolved from a common ancestral gene. They can give insights into the function and evolutionary processes.
- Phylogenomics: Using genome data to reconstruct the evolutionary histories and relationships among a group of species.
3. Functional Genomics:
Functional genomics aims to understand the function and interaction of genes and the proteins they encode. It involves a variety of methods to study gene expression, protein function, and interaction networks.
- Microarrays: These are tools used to measure the expression levels of thousands of genes simultaneously. By comparing gene expression profiles under different conditions or in different tissues, researchers can identify genes that play key roles in specific processes or diseases.
- RNA-seq: A next-generation sequencing method used to analyze the presence and quantity of RNA in a sample. It provides a more detailed and accurate picture of gene expression than microarrays.
- Protein-Protein Interaction (PPI) Networks: Techniques like yeast two-hybrid screening or tandem affinity purification are used to identify interactions between proteins, helping to elucidate complex cellular processes and pathways.
- Gene Knockout and Knockdown Experiments: By deactivating or reducing the expression of specific genes, researchers can study their function and the effects of their absence.
In summary, the advancements in genomics methods and technologies have transformed our understanding of genetics, evolution, and biology. These tools provide comprehensive views of genomes, allowing researchers to delve deeper into the intricacies of genetic information and its role in life.
Applications of Genomics
1. Identification of Genes Associated with Diseases:
One of the most impactful applications of genomics has been the identification of genes associated with various diseases. This has revolutionized our understanding of the genetic basis of many conditions and has paved the way for improved diagnostic, therapeutic, and preventive measures.
- Genetic Testing: By identifying mutations or variations in genes that are linked to specific diseases, genetic tests can be developed. These tests can diagnose conditions, predict the risk of developing a disease, or determine the likelihood of passing on a genetic disorder to offspring.
- Targeted Therapies: With the knowledge of specific genetic mutations that cause or contribute to a disease, drugs can be developed to target these mutations. For example, certain cancers are driven by specific genetic mutations, and drugs targeting these mutations have shown effectiveness in treating the disease.
- Understanding Disease Mechanisms: By identifying genes associated with diseases, researchers can understand the underlying mechanisms of these conditions, leading to the development of new treatment strategies.
2. Evolutionary Studies:
Genomics has provided a wealth of data that has been instrumental in understanding evolutionary relationships, processes, and adaptations.
- Phylogenomics: By comparing the genomes of different species, researchers can reconstruct the evolutionary history of organisms and determine their relationships.
- Tracing Evolutionary Adaptations: Genomic studies can identify genes or genetic changes that have provided specific evolutionary advantages. For instance, studying the genomes of high-altitude populations has revealed genetic adaptations related to oxygen utilization.
- Speciation Studies: Genomics can provide insights into how new species arise by identifying genetic changes that contribute to reproductive isolation.
3. Personalized Medicine:
The era of personalized medicine, where treatments and preventive measures are tailored to an individual’s genetic makeup, has been ushered in by advances in genomics.
- Pharmacogenomics: Some individuals respond differently to drugs due to their genetic makeup. By understanding these genetic differences, doctors can prescribe medications that are more effective and have fewer side effects for individual patients.
- Genomic Risk Assessment: By analyzing an individual’s genome, it’s possible to identify genetic variants that increase the risk of certain diseases. With this knowledge, individuals can take preventive measures or undergo regular screenings.
- Tailored Therapies: In conditions like cancer, genomic analyses can identify specific mutations driving the disease in an individual patient. Targeted therapies can then be prescribed based on the genetic profile of the tumor.
In conclusion, the applications of genomics are vast and transformative. From improving our understanding of diseases to personalizing medical treatments and unraveling the mysteries of evolution, genomics is at the forefront of modern biological research and medicine.
Proteomics
A. Definition and Overview
Proteomics is the comprehensive study of the entire set of proteins expressed by an organism. Proteins are the workhorses of the cell, executing a wide range of functions, from catalyzing metabolic reactions to serving as structural components. While genomics provides the blueprint of life with DNA sequences, proteomics delves into the dynamic world of proteins, revealing how they are expressed, modified, function, and interact within the cellular environment.
The primary objectives of proteomics include:
- Protein Identification: Determining the specific proteins that are present in a particular cell, tissue, or organism under specific conditions.
- Quantitative Proteomics: Measuring the relative or absolute abundance of proteins in different samples, which can be critical for understanding changes in cellular processes or disease states.
- Post-translational Modifications (PTMs): Studying modifications made to proteins after they are synthesized, such as phosphorylation, glycosylation, or ubiquitination. These modifications can dramatically affect protein function and activity.
- Protein-Protein Interactions: Uncovering the networks of protein interactions within a cell, providing insights into complex cellular processes and pathways.
- Structural Proteomics: Analyzing the three-dimensional structures of proteins to understand their functions, mechanisms, and potential interactions with other molecules.
At its core, proteomics focuses on protein expression, function, and interactions. By comprehensively analyzing the proteome—the entire set of proteins—researchers can gain insights into the functional networks of the cell, the mechanisms of diseases, and potential therapeutic targets. Proteomics complements genomics and provides a deeper understanding of cellular dynamics, mechanisms, and responses to various stimuli or conditions.
Methods and Technologies in Proteomics
1. Mass Spectrometry (MS):
Mass spectrometry is a central technology in proteomics that allows for the identification and quantification of proteins based on the mass-to-charge ratio of their ions. It is often used in conjunction with other techniques like liquid chromatography (LC-MS/MS) to separate and analyze complex protein mixtures.
- Protein Identification: By fragmenting proteins into peptides and analyzing their mass spectra, specific proteins can be identified based on their unique peptide sequences.
- Quantitative Mass Spectrometry: Techniques such as isobaric tags for relative and absolute quantitation (iTRAQ) and tandem mass tags (TMT) allow for the quantification of proteins in different samples.
- Analysis of Post-translational Modifications: MS can identify and quantify PTMs on proteins, providing insights into protein activity, localization, and interactions.
2. Two-dimensional Gel Electrophoresis (2D-GE):
2D-GE is a classic method used to separate proteins based on two properties: their isoelectric point (pI) and molecular weight.
- Isoelectric Focusing (IEF): In the first dimension, proteins are separated based on their pI, which is the pH at which a protein has no net charge.
- SDS-PAGE: In the second dimension, proteins are separated based on their molecular weight using polyacrylamide gel electrophoresis in the presence of a detergent (SDS).
The result is a gel with protein spots, where each spot corresponds to a unique protein. Spots can be excised, digested into peptides, and then identified using mass spectrometry.
3. Protein-Protein Interaction Studies:
Understanding the interactions between proteins is crucial for deciphering cellular processes and pathways.
- Yeast Two-Hybrid System: This genetic method is used to detect physical interactions between two proteins. One protein is fused to a DNA-binding domain and the other to a transcriptional activation domain. Interaction between the two proteins brings the domains together, activating the transcription of a reporter gene.
- Tandem Affinity Purification (TAP): This method involves tagging a protein of interest and expressing it in cells. The tagged protein and its interacting partners are then purified using a two-step affinity purification process, followed by identification using mass spectrometry.
- Co-immunoprecipitation (Co-IP): Proteins that interact with a protein of interest are co-precipitated using a specific antibody and then identified using Western blotting or mass spectrometry.
- Protein Microarrays: These are high-throughput methods where thousands of proteins are immobilized on a solid surface. They can be probed with labeled proteins, antibodies, or small molecules to study protein interactions, activity, or PTMs.
In conclusion, the combination of these methods and technologies provides a comprehensive toolkit for proteomic research. By leveraging these techniques, researchers can delve deep into the proteome, uncovering the intricate networks of protein expression, function, and interactions that underpin cellular life.
Applications of Proteomics
1. Drug Discovery and Development:
Proteomics plays a pivotal role in the pharmaceutical industry, aiding in the discovery of new drug targets and the development of therapeutic agents.
- Target Identification: By comparing the proteomes of diseased and healthy cells or tissues, researchers can identify proteins that are aberrantly expressed or modified in disease states. Such proteins can serve as potential drug targets.
- Biomarker Discovery: Proteomic analyses can identify proteins or protein modifications that correlate with disease states, disease progression, or therapeutic responses. These biomarkers can be used for early diagnosis, prognosis, or monitoring therapeutic efficacy.
- Pharmacoproteomics: This sub-field studies the effects of drugs on protein expression, modification, and interaction. It provides insights into drug mechanisms of action, potential side effects, and patient-specific responses.
- Drug Repurposing: Proteomic profiles can help identify new uses for existing drugs by revealing previously unknown protein targets or pathways affected by the drug.
2. Diagnosis and Treatment of Diseases:
Proteomics offers tools and insights for clinical applications, enhancing the diagnosis, prognosis, and treatment of various diseases.
- Disease Profiling: By analyzing the protein expression patterns in patient samples, diseases can be classified into subtypes, leading to more personalized and effective treatments.
- Therapeutic Protein Development: Proteomics can identify proteins or peptides with therapeutic potential, leading to the development of biologic drugs like monoclonal antibodies or recombinant proteins.
- Personalized Medicine: Proteomic profiles can predict how individual patients will respond to specific treatments, enabling tailored therapeutic strategies for maximum efficacy and minimal side effects.
3. Understanding Cellular Processes:
Proteomics offers a deep dive into the inner workings of cells, elucidating complex biological processes and pathways.
- Signal Transduction: By studying the proteomic changes in response to stimuli, researchers can map out signaling pathways and understand how cells perceive and respond to their environment.
- Protein Complexes and Networks: Proteomic methods, especially those studying protein-protein interactions, reveal the intricate networks of protein complexes that execute cellular functions.
- Cellular Stress Responses: Proteomic analyses can uncover how cells respond to various stresses, such as heat shock, oxidative stress, or drug exposure. This provides insights into cellular defense mechanisms and potential vulnerabilities.
- Post-translational Modifications (PTMs): Proteomics is instrumental in identifying and studying PTMs, which play critical roles in regulating protein activity, localization, and interactions.
In summary, the applications of proteomics span from bench to bedside, driving advancements in basic biology, clinical diagnostics, and therapeutic development. By studying the dynamic world of proteins, proteomics provides invaluable insights into health, disease, and the intricate dance of molecules that constitutes life.
Key Differences
A. Scope
Genomics:
- Focus: Genomics concentrates on the entire genome, which includes the complete set of DNA sequences in an organism.
- Level of Study: It operates at the DNA level, examining genes, regulatory elements, and non-coding sequences.
- Information Retrieved: Genomics provides the blueprint or genetic code of an organism, revealing potential instructions for cellular processes and functions. It can also give insights into evolutionary relationships and genetic predispositions.
Proteomics:
- Focus: Proteomics delves into the entire proteome, encompassing the full set of proteins expressed by an organism.
- Level of Study: It operates at the protein level, analyzing protein structures, functions, modifications, and interactions.
- Information Retrieved: Proteomics offers a dynamic view, showcasing the actual execution of genetic instructions. It reveals how proteins are produced, modified, interact, and participate in cellular processes. This field provides insights into cellular mechanisms, disease states, and potential therapeutic targets.
Complexity
Genomics:
- Nature of Information: Genomic information is static. Once an organism’s DNA is sequenced, its base pair sequence remains constant throughout its life.
- Implication: While DNA sequences are unchanging, their interpretation can evolve as our understanding of genetics grows. For instance, regions once considered “junk DNA” are now understood to have regulatory roles.
- Variability: While the DNA sequence itself is constant, how genes are expressed can vary. This gene expression is influenced by various factors, including environmental conditions, developmental stage, and external stimuli.
Proteomics:
- Nature of Information: Proteomic information is dynamic. Protein expression, modification, and interaction can change in response to numerous factors.
- Implication: This dynamism means that a single gene can give rise to multiple protein variants due to alternative splicing, post-translational modifications, and other processes. As a result, the proteome is more complex than the genome.
- Variability: Protein levels can vary widely based on cellular conditions, stressors, developmental stages, and diseases. This makes the study of proteomics more intricate as it seeks to capture a snapshot of the ever-changing cellular environment.
Technologies Used
Genomics:
- DNA Sequencers: These are instruments used to determine the precise sequence of nucleotides in a DNA sample. There have been several generations of DNA sequencers, from the early Sanger sequencers to modern next-generation sequencers that can rapidly sequence entire genomes.
- Microarrays: Also known as gene chips, microarrays are used to measure the expression levels of thousands of genes simultaneously. They consist of a solid surface where DNA probes are attached. These probes can hybridize with target sequences, allowing researchers to gauge the relative abundance of different genes in a sample.
Proteomics:
- Mass Spectrometers: Central to proteomics, mass spectrometers identify and quantify proteins based on the mass-to-charge ratio of their ions. They can determine the composition of individual proteins and peptides, enabling protein identification, quantification, and analysis of post-translational modifications.
- Chromatography: Chromatographic techniques, such as liquid chromatography (LC), are used in conjunction with mass spectrometry (LC-MS/MS) to separate complex protein mixtures before analysis. This separation ensures that individual proteins can be identified and quantified more accurately in a sample.
Data Output
Genomics:
- Sequence Data: One of the primary outputs of genomics is the DNA sequence data, which provides the linear order of nucleotides (A, T, C, G) in a given DNA sample. This sequence data forms the foundation for understanding genes, regulatory regions, and other genomic elements.
- Genomic Maps: These are spatial representations of genes and other features on a genome. Genomic maps can be of different types:
- Physical Maps: Show the physical distances between genes or other features, usually measured in base pairs.
- Genetic Maps (or Linkage Maps): Show the relative positions of genes based on how frequently they are inherited together. The distances are measured in centimorgans (cM), indicating the likelihood of recombination between genes.
Proteomics:
- Protein Profiles: Proteomics often results in a profile or spectrum of proteins present in a sample. This profile can show the abundance of each protein and can change depending on the condition, tissue, or time point studied. Quantitative proteomics can provide precise measurements of protein levels, and differential protein expression can highlight key players in specific biological processes or disease states.
- Interaction Networks: One of the goals of proteomics is to understand how proteins interact with each other. The data output can be in the form of interaction maps or networks, illustrating how proteins come together to form complexes, participate in pathways, or influence each other’s functions. These networks provide a holistic view of cellular processes and can reveal key nodes or hubs critical for cellular function.
Interrelationship
A. How Genomics Can Inform Proteomics and Vice Versa:
- From Genomics to Proteomics:
- Predictive Protein Mapping: Genomic data, specifically DNA sequences, provide the blueprints for proteins. By identifying open reading frames (ORFs) and coding sequences in genomic data, researchers can predict the potential proteins an organism can produce.
- Regulatory Insights: Genomic analyses can identify regulatory regions, such as promoters and enhancers, that control gene expression. Understanding these regulatory elements can provide insights into the conditions or signals that might induce or repress protein expression.
- Genetic Variants and Protein Function: Genomic studies can identify genetic variants like single nucleotide polymorphisms (SNPs). Some SNPs may lead to amino acid changes in proteins, potentially affecting their function, stability, or interaction partners. Proteomics can then be used to study the functional consequences of these genetic variations.
- From Proteomics to Genomics:
- Protein Complexity and Gene Annotations: Proteomic data, especially regarding alternative splicing and post-translational modifications, can refine gene annotations. Discovering multiple protein forms can lead to a re-evaluation of genomic data to identify previously overlooked exons or regulatory elements.
- Functional Annotation of Genes: Proteomic studies that elucidate the function, localization, or interaction partners of a protein can provide functional annotations for corresponding genes, enhancing the understanding of their roles in cellular processes.
B. Importance of Integrating Both Fields for Comprehensive Understanding:
- Holistic View of Cellular Processes: Genomics provides the blueprint, while proteomics reveals the machinery in action. By integrating both, researchers can achieve a comprehensive understanding of cellular processes from genetic instructions to functional outcomes.
- Disease Understanding and Treatment: Many diseases result from a combination of genetic predispositions and alterations in protein function or expression. By studying both the genome and proteome, researchers can identify disease mechanisms more precisely, leading to better diagnostic tools, therapeutic targets, and treatment strategies.
- Bridging the Genotype-Phenotype Gap: One of the central challenges in biology is connecting genetic information (genotype) with observable traits (phenotype). Proteins are key players in manifesting these traits. Integrating genomics and proteomics allows for a clearer pathway from genetic information to phenotypic outcomes.
- Systems Biology and Network Analysis: Modern biology increasingly adopts a systems perspective, looking at how various components of a cell or organism interact as a network. Integrating genomics and proteomics provides a rich dataset for constructing and analyzing these networks, leading to insights into emergent properties and system behaviors.
In essence, while both genomics and proteomics are powerful disciplines on their own, their true potential is unlocked when they are integrated, providing a multi-dimensional view of biology and medicine.
Conclusion
A. The Importance of Both Fields in Modern Biology and Medicine:
Genomics and proteomics, as pillars of molecular biology, have undeniably revolutionized our understanding of life at the molecular level. Genomics, with its focus on the complete set of DNA sequences, provides the foundational blueprint of life, revealing the genetic underpinnings of organisms. It has been instrumental in identifying genetic markers for diseases, understanding evolutionary relationships, and elucidating the genetic architecture of complex traits.
Proteomics, on the other hand, delves into the dynamic world of proteins – the primary executors of genetic instructions. It offers insights into protein structures, functions, interactions, and modifications, shedding light on cellular mechanisms and responses to various conditions. In medicine, proteomics has been pivotal in identifying disease biomarkers, understanding pathological processes, and developing targeted therapies.
B. The Potential of Combined Genomics and Proteomics Research in the Future:
The integration of genomics and proteomics promises a future of unprecedented advancements in biology and medicine. By combining the static blueprint of genomics with the dynamic insights of proteomics, researchers can achieve a holistic understanding of cellular and organismal processes.
- Personalized Medicine: The combined power of genomics and proteomics can tailor medical treatments to individual patients, considering both their genetic makeup and protein expression profiles. This approach can optimize therapeutic efficacy and minimize adverse effects.
- Complex Disease Understanding: Many diseases, especially chronic and multifactorial ones, result from intricate interactions between genes and proteins. Combined research can unravel these complexities, leading to better prevention, diagnosis, and treatment strategies.
- Evolutionary and Developmental Insights: Integrating genomics and proteomics can provide deeper insights into evolutionary processes, species diversification, and developmental mechanisms.
- Drug Discovery and Development: The combined data from both fields can accelerate the discovery of novel drug targets and the development of more effective therapeutic agents, especially biologics.
- Systems Biology: The future of biology lies in understanding organisms as integrated systems. The fusion of genomics and proteomics data can drive the construction of detailed and comprehensive biological networks, revealing emergent properties and system-level behaviors.
In conclusion, while genomics and proteomics individually have driven significant advancements in biology and medicine, their combined potential is vast and largely untapped. As technologies continue to advance and data integration becomes more sophisticated, the fusion of these disciplines will undoubtedly pave the way for groundbreaking discoveries and innovations in the coming decades.